Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions
- PMID: 29588129
- PMCID: PMC6005738
- DOI: 10.1016/j.tcb.2018.02.009
Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions
Abstract
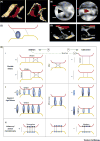
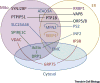
Interorganellar contacts are increasingly recognized as central to the control of cellular behavior. These contacts, which typically involve a small fraction of the endomembrane surface, are local communication hubs that resemble synapses. We propose the term contactology to denote the analysis of interorganellar contacts. Endoplasmic reticulum (ER) contacts with mitochondria were recognized several decades ago; major roles in ion and lipid transfer, signaling, and membrane dynamics have been established, while others continue to emerge. The functional diversity of ER-mitochondrial (ER-mito) contacts is mirrored in their structural heterogeneity, with subspecialization likely supported by multiple, different linker-forming protein structures. The nanoscale size of the contacts has made studying their structure, function, and dynamics difficult. This review focuses on the structure of the ER-mito contacts, methods for studying them, and the roles of contacts in Ca2+ and reactive oxygen species (ROS) signaling.
Keywords: IP3 receptor; calcium ion; linkers; mitochondrion-associated membrane; ryanodine receptor; sarcoplasmic reticulum.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

References
-
- Kerkhofs M, et al. Alterations in Ca2+ Signalling via ER-Mitochondria Contact Site Remodelling in Cancer. Advances in experimental medicine and biology. 2017;997:225–254. - PubMed
-
- Marchi S, et al. Endoplasmic Reticulum-Mitochondria Communication Through Ca2+ Signaling: The Importance of Mitochondria-Associated Membranes (MAMs) Advances in experimental medicine and biology. 2017;997:49–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

