Regulatory CD4+ T Cells Recognize Major Histocompatibility Complex Class II Molecule-Restricted Peptide Epitopes of Apolipoprotein B
- PMID: 29588316
- PMCID: PMC6160361
- DOI: 10.1161/CIRCULATIONAHA.117.031420
Regulatory CD4+ T Cells Recognize Major Histocompatibility Complex Class II Molecule-Restricted Peptide Epitopes of Apolipoprotein B
Abstract
Background: CD4+ T cells play an important role in atherosclerosis, but their antigen specificity is poorly understood. Immunization with apolipoprotein B (ApoB, core protein of low density lipoprotein) is known to be atheroprotective in animal models. Here, we report on a human APOB peptide, p18, that is sequence-identical in mouse ApoB and binds to both mouse and human major histocompatibility complex class II molecules.
Methods: We constructed p18 tetramers to detect human and mouse APOB-specific T cells and assayed their phenotype by flow cytometry including CD4 lineage transcription factors, intracellular cytokines, and T cell receptor activation. Apolipoprotein E-deficient ( Apoe-/-) mice were vaccinated with p18 peptide or adjuvants alone, and atherosclerotic burden in the aorta was determined.
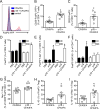
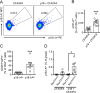
Results: In human peripheral blood mononuclear cells from donors without cardiovascular disease, p18 specific CD4+ T cells detected by a new human leukocyte antigen-antigen D related-p18 tetramers were mostly Foxp3+ regulatory T cells (Tregs). Donors with subclinical cardiovascular disease as detected by carotid artery ultrasound had Tregs coexpressing retinoic acid-related orphan receptor gamma t or T-bet, which were both almost absent in donors without cardiovascular disease. In Apoe-/- mice, immunization with p18 induced Tregs and reduced atherosclerotic lesions. After peptide restimulation, responding CD4+ T cells identified by Nur77-GFP (green fluorescent protein) were highly enriched in Tregs. A new mouse I-Ab-p18 tetramer identified the expansion of p18-specific CD4+ T cells on vaccination, which were enriched for interleukin-10-producing Tregs.
Conclusions: These findings show that APOB p18-specific CD4+ T cells are mainly Tregs in healthy donors, but coexpress other CD4 lineage transcription factors in donors with subclinical cardiovascular disease. This study identifies ApoB peptide 18 as the first Treg epitope in human and mouse atherosclerosis.
Keywords: antigen specificity; apoB-100; atherosclerosis; regulatory T cells; vaccination.
Conflict of interest statement
K.L. is an inventor on a patent application that includes p18. Other authors do not report any conflict of interest.
Figures




References
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI031834/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- R01 HL095140/HL/NHLBI NIH HHS/United States
- P01 HL136275/HL/NHLBI NIH HHS/United States
- R01 HL083760/HL/NHLBI NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- U01 AI103401/AI/NIAID NIH HHS/United States
- U01 AI103408/AI/NIAID NIH HHS/United States
- R01 HL121697/HL/NHLBI NIH HHS/United States
- U01 AI103397/AI/NIAID NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- R01 HL126543/HL/NHLBI NIH HHS/United States
- U01 AI103390/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- K01 HL137557/HL/NHLBI NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

