A cassette of basic amino acids in histone H2B regulates nucleosome dynamics and access to DNA damage
- PMID: 29588367
- PMCID: PMC5949990
- DOI: 10.1074/jbc.RA117.000358
A cassette of basic amino acids in histone H2B regulates nucleosome dynamics and access to DNA damage
Abstract
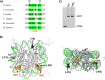
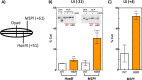
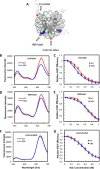
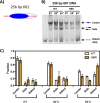
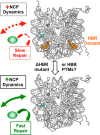
Nucleosome dynamics, such as spontaneous DNA unwrapping, are postulated to have a critical role in regulating the access of DNA repair machinery to DNA lesions within nucleosomes. However, the specific histone domains that regulate nucleosome dynamics and the impact of such changes in intrinsic nucleosome dynamics on DNA repair are not well understood. Previous studies identified a highly conserved region in the N-terminal tail of histone H2B known as the
Keywords: DNA repair; HBR domain; base excision repair (BER); chromatin; glycosylase; histone H2B repression; histone modification; nucleosome; polymerase beta.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

