Phospholipid subcellular localization and dynamics
- PMID: 29588369
- PMCID: PMC5925819
- DOI: 10.1074/jbc.R117.000582
Phospholipid subcellular localization and dynamics
Abstract
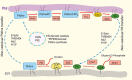
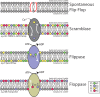
Membrane biology seeks to understand how lipids and proteins within bilayers assemble into large structures such as organelles and the plasma membranes. Historically, lipids were thought to merely provide structural support for bilayer formation and membrane protein function. Research has now revealed that phospholipid metabolism regulates nearly all cellular processes. Sophisticated techniques helped identify >10,000 lipid species suggesting that lipids support many biological processes. Here, we highlight the synthesis of the most abundant glycerophospholipid classes and their distribution in organelles. We review vesicular and nonvesicular transport pathways shuttling lipids between organelles and discuss lipid regulators of membrane trafficking and second messengers in eukaryotic cells.
Keywords: flippase; glycerophospholipid; lipid transfer proteins; membrane contact sites; membrane trafficking; nonvesicular transport; organelle; phosphatidylinositol; phospholipase; phospholipid metabolism; phospholipids; scramblase; sphingolipid; vesicular transport.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Tanford C. (1987) Amphiphile orientation: physical chemistry and biological function. Biochem. Soc. Trans. 15, Suppl, 1–7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

