Structure and Distribution of an Unrecognized Interstitium in Human Tissues
- PMID: 29588511
- PMCID: PMC5869738
- DOI: 10.1038/s41598-018-23062-6
Structure and Distribution of an Unrecognized Interstitium in Human Tissues
Erratum in
-
Author Correction: Structure and Distribution of an Unrecognized Interstitium in Human Tissues.Sci Rep. 2018 May 10;8(1):7610. doi: 10.1038/s41598-018-25732-x. Sci Rep. 2018. PMID: 29743629 Free PMC article.
Abstract
Confocal laser endomicroscopy (pCLE) provides real-time histologic imaging of human tissues at a depth of 60-70 μm during endoscopy. pCLE of the extrahepatic bile duct after fluorescein injection demonstrated a reticular pattern within fluorescein-filled sinuses that had no known anatomical correlate. Freezing biopsy tissue before fixation preserved the anatomy of this structure, demonstrating that it is part of the submucosa and a previously unappreciated fluid-filled interstitial space, draining to lymph nodes and supported by a complex network of thick collagen bundles. These bundles are intermittently lined on one side by fibroblast-like cells that stain with endothelial markers and vimentin, although there is a highly unusual and extensive unlined interface between the matrix proteins of the bundles and the surrounding fluid. We observed similar structures in numerous tissues that are subject to intermittent or rhythmic compression, including the submucosae of the entire gastrointestinal tract and urinary bladder, the dermis, the peri-bronchial and peri-arterial soft tissues, and fascia. These anatomic structures may be important in cancer metastasis, edema, fibrosis, and mechanical functioning of many or all tissues and organs. In sum, we describe the anatomy and histology of a previously unrecognized, though widespread, macroscopic, fluid-filled space within and between tissues, a novel expansion and specification of the concept of the human interstitium.
Conflict of interest statement
Dr. Theise has received travel sponsorship for attendance at a Mauna Kea Technology sponsored scientific conference. Dr. Benias has received sponsorship for attendance at a Mauna Kea Technology sponsored scientific conference. Dr Carr-Locke has received royalties from US Endoscopy and Telemed Systems, has been a consultant for Boston Scientific Endoscopy, Cook Medical, EndoChoice, Mauna Kea Technologies, and Olympus Corporation, and holds a patent with ValenTx.
Figures
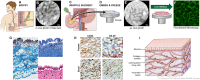



Comment in
-
The Interstitial Space Takes Shape.Hepatology. 2019 Apr;69(4):1830-1832. doi: 10.1002/hep.30268. Epub 2019 Feb 17. Hepatology. 2019. PMID: 30215854 No abstract available.
References
-
- Aukland K. Distribution of body fluids: local mechanisms guarding interstitial fluid volume. J. Physiol. (Paris) 1984;79:395–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

