A fast and sensitive activity assay for lytic polysaccharide monooxygenase
- PMID: 29588664
- PMCID: PMC5865291
- DOI: 10.1186/s13068-018-1063-6
A fast and sensitive activity assay for lytic polysaccharide monooxygenase
Abstract
Background: Lytic polysaccharide monooxygenases (LPMO) release a spectrum of cleavage products from their polymeric substrates cellulose, hemicellulose, or chitin. The correct identification and quantitation of these released products is the basis of MS/HPLC-based detection methods for LPMO activity. The duration, effort, and intricate analysis allow only specialized laboratories to measure LPMO activity in day-to-day work. A spectrophotometric assay will simplify the screening for LPMO in culture supernatants, help monitor recombinant LPMO expression and purification, and support enzyme characterization.
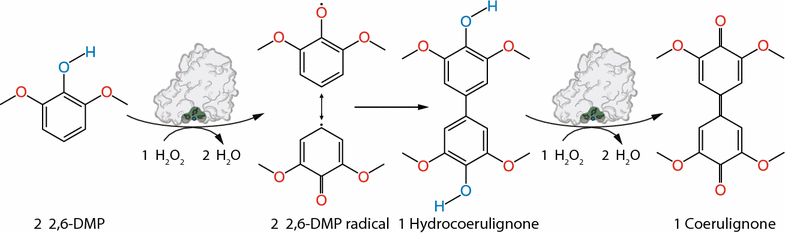
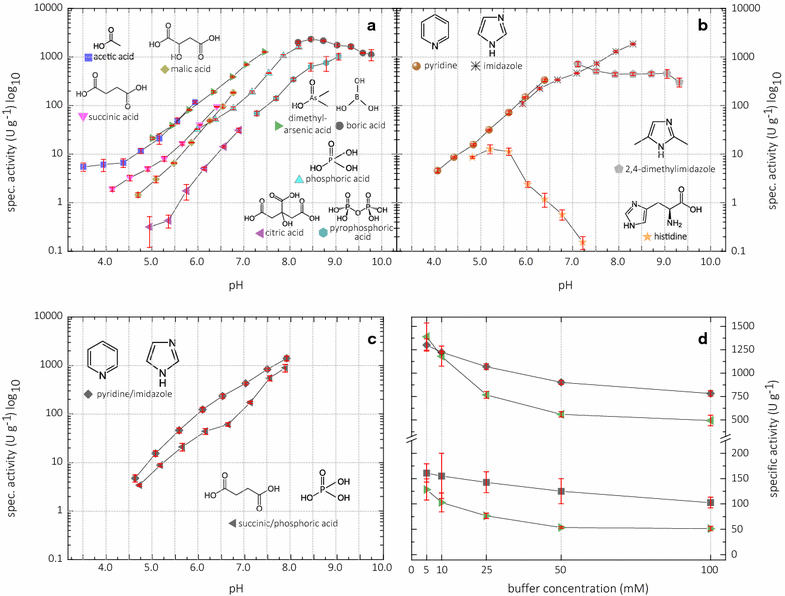
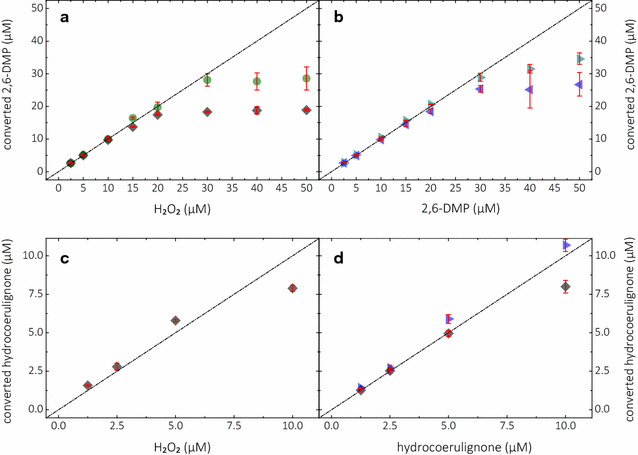
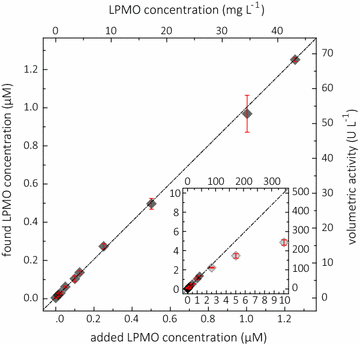
Results: Based on a newly discovered peroxidase activity of LPMO, we propose a fast, robust, and sensitive spectrophotometric activity assay using 2,6-dimethoxyphenol (2,6-DMP) and H2O2. The fast enzymatic assay (300 s) consists of 1 mM 2,6-DMP as chromogenic substrate, 100 µM H2O2 as cosubstrate, and an adequate activity of LPMO in a suitable buffer. The high molar absorption coefficient of the formed product coerulignone (ε469 = 53,200 M-1 cm-1) makes the assay sensitive and allows reliable activity measurements of LPMO in concentrations of approx. 0.5-50 mg L-1.
Conclusions: The activity assay based on 2,6-DMP detects a novel peroxidase activity of LPMO. This activity can be accurately measured and used for enzyme screening, production, and purification, and can also be applied to study binding constants or thermal stability. However, the assay has to be used with care in crude extracts, because other enzymes such as laccase or peroxidase will interfere with the assay. We also want to stress that the peroxidase activity is a homogeneous reaction with soluble substrates and should not be correlated to heterogeneous LPMO activity on polymeric substrates.
Keywords: 2,6-Dimethoxyphenol; Activity assay; Biomass degradation; Hydrogen peroxide; Lytic polysaccharide monooxygenase; Peroxidase activity.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

