Challenging current views on bile acid diarrhoea and malabsorption
- PMID: 29588835
- PMCID: PMC5868445
- DOI: 10.1136/flgastro-2017-100808
Challenging current views on bile acid diarrhoea and malabsorption
Abstract
Background: In 2012, the National Institute for Health and Care Excellence (NICE) assessed guidance (DG7) on the use of tauroselcholic (75selenium) acid (also known as SeHCAT) for the investigation of diarrhoea due to bile acid malabsorption (BAM) in patients with IBS-D and in patients with Crohn's disease who have not had an ileal resection. NICE concluded that tauroselcholic (75selenium) acid was recommended for use in research only. NICE will be reviewing the decision to update the guidance for tauroselcholic (75selenium) acid, for these populations, in March 2017.
Aim: Our aim is to summarise advances in BAM, also known as bile acid diarrhoea (BAD), and encourage clinicians to re-evaluate their understanding of this disorder.
Approach: We review the prevalence, diagnosis and treatment of BAD/BAM. We describe the new evidence available since the original NICE review in 2012, and discuss the economic issues associated with failure to diagnose or to treat BAD/BAM accurately.
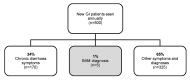
Evidence update: There is new and compelling evidence available since DG7, which shows that tauroselcholic (75selenium) acid scanning is a powerful tool in the diagnosis of BAD/BAM. We summarise published prevalence data (approximately 1% prevalence in the UK, as suggested by clinical practice diagnosis rates), and highlight that the true prevalence of BAD/BAM could be far greater than this.
Conclusion: We present evidence that challenges current opinion about this disorder, and we commend both clinicians and health technology assessment (HTA) agencies for being open to arguments and new evidence in any future HTAs.
Keywords: bile; cost-effectiveness; diarrhoea; malabsorption.
Conflict of interest statement
Competing interests: Financial support for this study was provided by General Electric (GE) Healthcare. The funding agreement ensured the authors’ independence in designing the study, interpreting the literature and writing the manuscript. The authors AD, ET and RA are employed by BresMed, which was reimbursed by GE Healthcare as a consultancy for its time developing the manuscript. JA reports personal fees and non-financial support from GE Healthcare, from Sanofi Aventis, during the conduct of the study. MK has no potential conflicts to disclose.
Figures
References
-
- Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol 2010;3:349–57. doi:10.1177/1756283X10377126 - DOI - PMC - PubMed
-
- Pattni SS, Brydon WG, Dew T, et al. . Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther 2013;38:967–76. doi:10.1111/apt.12466 - DOI - PubMed
-
- Brand SJ SW. Textbook of Gastroenterology. Philadelphia: JB Lippincott Company, 1995:25–71.
-
- Gupta A, Muls AC, Lalji A, et al. . Outcomes from treating bile acid malabsorption using a multidisciplinary approach. Support Care Cancer 2015;23:2881–90. doi:10.1007/s00520-015-2653-5 - DOI - PubMed
-
- Phillips F, Muls AC, Lalji A, et al. . Are bile acid malabsorption and bile acid diarrhoea important causes of loose stool complicating Cancer therapy? Colorectal Dis 2015;17:730–4. doi:10.1111/codi.12932 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous