Prospective International Randomized Phase II Study of Low-Dose Abiraterone With Food Versus Standard Dose Abiraterone In Castration-Resistant Prostate Cancer
- PMID: 29590007
- PMCID: PMC5941614
- DOI: 10.1200/JCO.2017.76.4381
Prospective International Randomized Phase II Study of Low-Dose Abiraterone With Food Versus Standard Dose Abiraterone In Castration-Resistant Prostate Cancer
Abstract
Purpose Abiraterone acetate (AA) is a standard of care for metastatic castration-resistant prostate cancer (CRPC). Despite a large food effect, AA was administered under fasting conditions in its pivotal trials. We sought to test the hypothesis that low-dose AA (LOW; 250 mg with a low-fat meal) would have comparable activity to standard AA (STD; 1,000 mg fasting) in patients with CRPC. Patients and Methods Patients (n = 72) with progressive CRPC from seven institutions in the United States and Singapore were randomly assigned to STD or LOW. Both arms received prednisone 5 mg twice daily. Prostate-specific antigen (PSA) was assessed monthly, and testosterone/dehydroepiandrosterone sulfate were assessed every 12 weeks with disease burden radiographic assessments. Plasma was collected for drug concentrations. Log change in PSA, as a pharmacodynamic biomarker for efficacy, was the primary end point, using a noninferiority design. Progression-free survival (PFS), PSA response (≥ 50% reduction), change in androgen levels, and pharmacokinetics were secondary end points. Results Thirty-six patients were accrued to both arms. At 12 weeks, there was a greater effect on PSA in the LOW arm (mean log change, -1.59) compared with STD (-1.19), and noninferiority of LOW was established according to predefined criteria. The PSA response rate was 58% in LOW and 50% in STD, and the median PFS was approximately 9 months in both groups. Androgen levels decreased similarly in both arms. Although there was no difference in PSA response or PFS, abiraterone concentrations were higher in STD. Conclusion Low-dose AA (with low-fat breakfast) is noninferior to standard dosing with respect to PSA metrics. Given the pharmacoeconomic implications, these data warrant consideration by prescribers, payers, and patients. Additional studies are indicated to assess the long-term efficacy of this approach.
Trial registration: ClinicalTrials.gov NCT01543776.
Figures


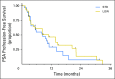

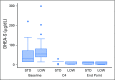
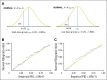

Comment in
-
Low-Fat Abiraterone Food Effect Is of Little Consequence.J Clin Oncol. 2018 May 10;36(14):1385-1386. doi: 10.1200/JCO.2018.78.0684. Epub 2018 Mar 28. J Clin Oncol. 2018. PMID: 29590006 No abstract available.
-
Low-Fat Abiraterone Food Effect Is of Great Consequence.J Clin Oncol. 2018 Oct 20;36(30):3058-3059. doi: 10.1200/JCO.2018.79.2358. Epub 2018 Sep 6. J Clin Oncol. 2018. PMID: 30188783 No abstract available.
-
Reply to I.F. Tannock, P. Isaacsson Velho et al, M. Tiako Meyo et al, and F.J.S.H. Woei-A-Jin et al.J Clin Oncol. 2018 Oct 20;36(30):3064-3065. doi: 10.1200/JCO.2018.79.3190. Epub 2018 Sep 6. J Clin Oncol. 2018. PMID: 30188784 No abstract available.
-
Low-Dose Abiraterone With Food: Rebutting an Editorial.J Clin Oncol. 2018 Oct 20;36(30):3060-3061. doi: 10.1200/JCO.2018.79.3018. Epub 2018 Sep 6. J Clin Oncol. 2018. PMID: 30188785 No abstract available.
-
There Is Now Compelling Evidence to Further Evaluate Lower Doses of Abiraterone Acetate in Men With Metastatic Prostate Cancer: It Should Be Safer, May Be as Effective and Less Expensive.J Clin Oncol. 2018 Oct 20;36(30):3059-3060. doi: 10.1200/JCO.2018.79.3166. Epub 2018 Sep 6. J Clin Oncol. 2018. PMID: 30188787 No abstract available.
-
Low-Dose Abiraterone Regimen: Drug Monitoring Might Be the Key.J Clin Oncol. 2018 Oct 20;36(30):3061-3062. doi: 10.1200/JCO.2018.79.3174. Epub 2018 Sep 6. J Clin Oncol. 2018. PMID: 30188788 No abstract available.
-
Dose Reduction May Jeopardize Efficacy of Abiraterone Acetate.J Clin Oncol. 2018 Oct 20;36(30):3062-3064. doi: 10.1200/JCO.2018.79.3182. Epub 2018 Sep 6. J Clin Oncol. 2018. PMID: 30188790 No abstract available.
-
Reply to I.F. Tannock, P. Isaacsson Velho et al, R.Z. Szmulewitz et al, M. Tiako Meyo et al, and F.J.S.H. Woei-A-Jin et al.J Clin Oncol. 2018 Oct 20;36(30):3065-3066. doi: 10.1200/JCO.2018.79.3208. Epub 2018 Sep 6. J Clin Oncol. 2018. PMID: 30188791 No abstract available.
References
-
- Fizazi K, Tran N, Fein L, et al. : Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352-360, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

