Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1
- PMID: 29590092
- PMCID: PMC6047741
- DOI: 10.1038/nature25986
Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1
Abstract
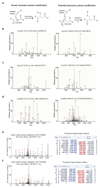
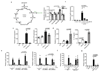
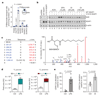
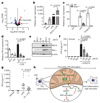
The endogenous metabolite itaconate has recently emerged as a regulator of macrophage function, but its precise mechanism of action remains poorly understood. Here we show that itaconate is required for the activation of the anti-inflammatory transcription factor Nrf2 (also known as NFE2L2) by lipopolysaccharide in mouse and human macrophages. We find that itaconate directly modifies proteins via alkylation of cysteine residues. Itaconate alkylates cysteine residues 151, 257, 288, 273 and 297 on the protein KEAP1, enabling Nrf2 to increase the expression of downstream genes with anti-oxidant and anti-inflammatory capacities. The activation of Nrf2 is required for the anti-inflammatory action of itaconate. We describe the use of a new cell-permeable itaconate derivative, 4-octyl itaconate, which is protective against lipopolysaccharide-induced lethality in vivo and decreases cytokine production. We show that type I interferons boost the expression of Irg1 (also known as Acod1) and itaconate production. Furthermore, we find that itaconate production limits the type I interferon response, indicating a negative feedback loop that involves interferons and itaconate. Our findings demonstrate that itaconate is a crucial anti-inflammatory metabolite that acts via Nrf2 to limit inflammation and modulate type I interferons.
Conflict of interest statement
The authors declare no competing interests.
Figures

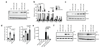

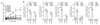





Comment in
-
Immunometabolism: Itaconate helps KEAP1's cool.Nat Rev Immunol. 2018 May;18(5):294-295. doi: 10.1038/nri.2018.27. Epub 2018 Apr 9. Nat Rev Immunol. 2018. PMID: 29629713 No abstract available.
-
A new BEACON of hope for the treatment of inflammation? The endogenous metabolite itaconate as an alternative activator of the KEAP1-Nrf2 system.Kidney Int. 2018 Oct;94(4):646-649. doi: 10.1016/j.kint.2018.07.018. Kidney Int. 2018. PMID: 30243306 No abstract available.
References
-
- Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12022/6/MRC_/Medical Research Council/United Kingdom
- 205455/WT_/Wellcome Trust/United Kingdom
- MC_U105663139/MRC_/Medical Research Council/United Kingdom
- PG/15/44/31574/BHF_/British Heart Foundation/United Kingdom
- MC_U105663142/MRC_/Medical Research Council/United Kingdom
- 110158/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- 18644/CRUK_/Cancer Research UK/United Kingdom
- FS/18/38/33659/BHF_/British Heart Foundation/United Kingdom
- MC_UU_00015/7/MRC_/Medical Research Council/United Kingdom
- MC_UU_00015/3/MRC_/Medical Research Council/United Kingdom
- 109443/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_00015/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

