Association between six-minute walk distance and long-term outcomes in patients with pulmonary arterial hypertension: Data from the randomized SERAPHIN trial
- PMID: 29590122
- PMCID: PMC5873992
- DOI: 10.1371/journal.pone.0193226
Association between six-minute walk distance and long-term outcomes in patients with pulmonary arterial hypertension: Data from the randomized SERAPHIN trial
Abstract
Background: Patients with pulmonary arterial hypertension who achieve a six-minute walk distance of 380-440 m may have improved prognosis. Using the randomized controlled trial of macitentan in pulmonary arterial hypertension (SERAPHIN), the association between six-minute walk distance and long-term outcomes was explored.
Methods: Patients with six-minute walk distance data at Month 6 were dichotomized as above or below the median six-minute walk distance (400 m) and assessed for future risk of pulmonary arterial hypertension-related death or hospitalization and all-cause death. Additionally, six-minute walk distance values at baseline, Month 6 and the change from baseline to Month 6 were categorized by quartiles. All associations were analyzed by the Kaplan-Meier method using a log-rank test and Cox regression models.
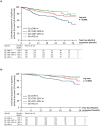
Results: Patients with a six-minute walk distance >400 m vs. ≤400 m at Month 6 have a reduced risk of pulmonary arterial hypertension-related death or hospitalization (hazard ratio 0.48; 95% confidence interval 0.33-0.69). The risk was also lower for patients with higher quartiles of six-minute walk distance at baseline or Month 6 (baseline: hazard ratio [Q4 (>430 m) vs. Q1 (≤300 m)] 0.23; 95% confidence interval 0.15-0.36; Month 6: hazard ratio [Q4 (>455 m) vs. Q1 (≤348 m)] 0.33; 95% confidence interval 0.19-0.55). In contrast, six-minute walk distance changes at Month 6 were not associated with the risk of pulmonary arterial hypertension-related death or hospitalization (p = 0.477). These findings were consistent when adjusted for known confounders. Similar results were observed for the risk of all-cause death up to end of study.
Conclusions: Patients with pulmonary arterial hypertension walking >400 m had better long-term prognosis. Although changes in six-minute walk distance were not associated with long-term outcomes, assessing absolute six-minute walk distance values remains important in the clinical management of patients with pulmonary arterial hypertension.
Conflict of interest statement
Figures



References
-
- Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. Journal of the American College of Cardiology. 2013;62(25 Suppl):D42–50. Epub 2013/12/21. doi: 10.1016/j.jacc.2013.10.032 . - DOI - PubMed
-
- McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, et al. Pulmonary arterial hypertension: epidemiology and registries. Journal of the American College of Cardiology. 2013;62(25 Suppl):D51–9. Epub 2013/12/21. doi: 10.1016/j.jacc.2013.10.023 . - DOI - PubMed
-
- Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2013;62(25 Suppl):D60–72. Epub 2013/12/21. doi: 10.1016/j.jacc.2013.10.031 . - DOI - PubMed
-
- Deboeck G, Taboada D, Hagan G, Treacy C, Page K, Sheares K, et al. Maximal cardiac output determines 6 minutes walking distance in pulmonary hypertension. PloS one. 2014;9(3):e92324 Epub 2014/03/22. doi: 10.1371/journal.pone.0092324 - DOI - PMC - PubMed
-
- Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161(2 Pt 1):487–92. Epub 2000/02/15. doi: 10.1164/ajrccm.161.2.9906015 . - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

