The neurochemistry and morphology of functionally identified corneal polymodal nociceptors and cold thermoreceptors
- PMID: 29590195
- PMCID: PMC5874071
- DOI: 10.1371/journal.pone.0195108
The neurochemistry and morphology of functionally identified corneal polymodal nociceptors and cold thermoreceptors
Abstract
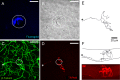
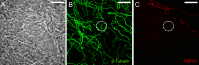
It is generally believed that the unencapsulated sensory nerve terminals of modality specific C- and Aδ-neurons lack structural specialization. Here we determined the morphology of functionally defined polymodal receptors and cold thermoreceptors in the guinea pig corneal epithelium. Polymodal receptors and cold thermoreceptors were identified by extracellular recording at the surface of the corneal epithelium. After marking the recording sites, corneas were processed to reveal immunoreactivity for the transient receptor potential channels TRPV1 (transient receptor potential cation channel, subfamily V, member 1) or TPRM8 (transient receptor potential cation channel subfamily M member 8). Polymodal receptor nerve terminals (n = 6) were TRPV1-immunoreactive and derived from an axon that ascended from the sub-basal plexus to the squamous cell layer where it branched into fibers that ran parallel to the corneal surface and terminated with small bulbar endings (ramifying endings). Cold thermoreceptor nerve terminals were TRPM8-immunoreactive (n = 6) and originated from an axon that branched as it ascended through the wing cell and squamous cell layers and terminated with large bulbar endings (complex endings). These findings indicate that modality specific corneal sensory neurons with unencapsulated nerve endings have distinct nerve terminal morphologies that are likely to relate to their function.
Conflict of interest statement
Figures




References
-
- Owens DM, Lumpkin EA. Diversification and specialization of touch receptors in skin. Cold Spring Harb Perspect Med. 2014; 4(6). doi: 10.1101/cshperspect.a013656 - DOI - PMC - PubMed
-
- Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346(6212):950–954. doi: 10.1126/science.1254229 - DOI - PMC - PubMed
-
- Hensel H, Andres KH, von During M. Structure and function of cold receptors. Pflugers Archiv: Eur J Physiol. 1974; 352: 1–10. - PubMed
-
- Heppelmann B, Gallar J, Trost B, Schmidt RF, Belmonte C. Three-dimensional reconstruction of scleral cold thermoreceptors of the cat eye. J Comp Neurol. 2001; 44: 148–154. - PubMed
-
- Li L, Ginty DD. The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles. Elife. 2014; 3: e01901 doi: 10.7554/eLife.01901 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

