Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development
- PMID: 29590310
- PMCID: PMC6134206
- DOI: 10.1093/biolre/ioy072
Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development
Abstract
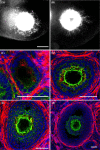
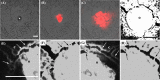
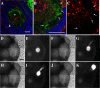
Proper development and maturation of oocytes requires interaction with granulosa cells. Previous reports have indicated that mammalian oocytes connect with cumulus cells through gap junctions at the tip of transzonal projections that extend from the cells. Although the gap junctions between oocytes and transzonal projections provide a pathway through which small molecules (<1 kDa) can travel, it is unclear how molecules >1 kDa are transported between the oocytes and cumulus cells. In this study, we presented new connections between oocytes and granulosa cells. The green fluorescein protein Aequorea coerulescens green fluorescein protein (AcGFP1) localizing in oocyte cell membrane, 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate and dextran conjugates (10,000 MW) injected into the oocytes, which were unable to pass through gap junctions, were diffused from the oocytes into the surrounding granulosa cells through these connections. These connect an oocyte to the surrounding cumulus and granulosa cells by fusing with the cell membranes and forming a large complex during follicle development. Furthermore, we show two characteristics of these connections during follicle development-the localization of growth and differentiation factor-9 within the connections and the dynamics of the connections at ovulation. This article presents for the first time that mammalian oocytes directly connect to granulosa cells by fusing with the cell membrane, similar to that in Drosophila.
Figures



Similar articles
-
Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle.Sci Rep. 2019 Feb 4;9(1):1262. doi: 10.1038/s41598-018-37766-2. Sci Rep. 2019. PMID: 30718581 Free PMC article.
-
Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence.Dev Biol. 2000 Oct 15;226(2):167-79. doi: 10.1006/dbio.2000.9863. Dev Biol. 2000. PMID: 11023678
-
Cellular basis for paracrine regulation of ovarian follicle development.Reproduction. 2001 May;121(5):647-53. doi: 10.1530/rep.0.1210647. Reproduction. 2001. PMID: 11427152 Review.
-
Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation.Dev Biol. 1990 Aug;140(2):307-17. doi: 10.1016/0012-1606(90)90081-s. Dev Biol. 1990. PMID: 2115479
-
Oocyte-somatic cell interactions during follicle development in mammals.Anim Reprod Sci. 2004 Jul;82-83:431-46. doi: 10.1016/j.anireprosci.2004.05.017. Anim Reprod Sci. 2004. PMID: 15271471 Review.
Cited by
-
Activation-induced cytidine deaminase is a possible regulator of cross-talk between oocytes and granulosa cells through GDF-9 and SCF feedback system.Sci Rep. 2021 Feb 15;11(1):3833. doi: 10.1038/s41598-021-83529-x. Sci Rep. 2021. PMID: 33589683 Free PMC article.
-
TMT-based proteomic and bioinformatic analyses of human granulosa cells from obese and normal-weight female subjects.Reprod Biol Endocrinol. 2021 May 20;19(1):75. doi: 10.1186/s12958-021-00760-x. Reprod Biol Endocrinol. 2021. PMID: 34016141 Free PMC article.
-
Short-term Western-style diet negatively impacts reproductive outcomes in primates.JCI Insight. 2021 Feb 22;6(4):e138312. doi: 10.1172/jci.insight.138312. JCI Insight. 2021. PMID: 33616080 Free PMC article.
-
Metabolomics analysis of follicular fluid coupled with oocyte aspiration reveals importance of glucocorticoids in primate periovulatory follicle competency.Sci Rep. 2021 Mar 22;11(1):6506. doi: 10.1038/s41598-021-85704-6. Sci Rep. 2021. PMID: 33753762 Free PMC article.
-
Intercellular communication in the cumulus-oocyte complex during folliculogenesis: A review.Front Cell Dev Biol. 2023 Jan 19;11:1087612. doi: 10.3389/fcell.2023.1087612. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36743407 Free PMC article. Review.
References
-
- Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod 2000; 5:143–152. - PubMed
-
- Ge L, Han D, Lan GC, Zhou P, Liu Y, Zhang X, Sui HS, Tan JH. Factors affecting the in vitro action of cumulus cells on the maturing mouse oocytes. Mol Reprod Dev 2008; 75:136–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

