Duration of Adjuvant Chemotherapy for Stage III Colon Cancer
- PMID: 29590544
- PMCID: PMC6426127
- DOI: 10.1056/NEJMoa1713709
Duration of Adjuvant Chemotherapy for Stage III Colon Cancer
Abstract
Background: Since 2004, a regimen of 6 months of treatment with oxaliplatin plus a fluoropyrimidine has been standard adjuvant therapy in patients with stage III colon cancer. However, since oxaliplatin is associated with cumulative neurotoxicity, a shorter duration of therapy could spare toxic effects and health expenditures.
Methods: We performed a prospective, preplanned, pooled analysis of six randomized, phase 3 trials that were conducted concurrently to evaluate the noninferiority of adjuvant therapy with either FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) administered for 3 months, as compared with 6 months. The primary end point was the rate of disease-free survival at 3 years. Noninferiority of 3 months versus 6 months of therapy could be claimed if the upper limit of the two-sided 95% confidence interval of the hazard ratio did not exceed 1.12.
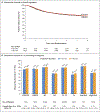
Results: After 3263 events of disease recurrence or death had been reported in 12,834 patients, the noninferiority of 3 months of treatment versus 6 months was not confirmed in the overall study population (hazard ratio, 1.07; 95% confidence interval [CI], 1.00 to 1.15). Noninferiority of the shorter regimen was seen for CAPOX (hazard ratio, 0.95; 95% CI, 0.85 to 1.06) but not for FOLFOX (hazard ratio, 1.16; 95% CI, 1.06 to 1.26). In an exploratory analysis of the combined regimens, among the patients with T1, T2, or T3 and N1 cancers, 3 months of therapy was noninferior to 6 months, with a 3-year rate of disease-free survival of 83.1% and 83.3%, respectively (hazard ratio, 1.01; 95% CI, 0.90 to 1.12). Among patients with cancers that were classified as T4, N2, or both, the disease-free survival rate for a 6-month duration of therapy was superior to that for a 3-month duration (64.4% vs. 62.7%) for the combined treatments (hazard ratio, 1.12; 95% CI, 1.03 to 1.23; P=0.01 for superiority).
Conclusions: Among patients with stage III colon cancer receiving adjuvant therapy with FOLFOX or CAPOX, noninferiority of 3 months of therapy, as compared with 6 months, was not confirmed in the overall population. However, in patients treated with CAPOX, 3 months of therapy was as effective as 6 months, particularly in the lower-risk subgroup. (Funded by the National Cancer Institute and others.).
Figures

Comment in
-
A New IDEA in Adjuvant Chemotherapy for Colon Cancer.N Engl J Med. 2018 Mar 29;378(13):1242-1244. doi: 10.1056/NEJMe1800419. N Engl J Med. 2018. PMID: 29590539 No abstract available.
-
Duration of Adjuvant Chemotherapy for Stage III Colon Cancer.N Engl J Med. 2018 Jul 26;379(4):395. doi: 10.1056/NEJMc1805498. N Engl J Med. 2018. PMID: 30048061 No abstract available.
-
Duration of Adjuvant Chemotherapy for Stage III Colon Cancer.N Engl J Med. 2018 Jul 26;379(4):395-6. doi: 10.1056/NEJMc1805498. N Engl J Med. 2018. PMID: 30048062 No abstract available.
References
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51. - PubMed
-
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–16. - PubMed
-
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011; 29: 1465–71. - PubMed
-
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007; 25:2198–204. - PubMed
-
- André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015; 33: 4176–87. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
