Leucine Zipper-Bearing Kinase Is a Critical Regulator of Astrocyte Reactivity in the Adult Mammalian CNS
- PMID: 29590625
- PMCID: PMC5905706
- DOI: 10.1016/j.celrep.2018.02.102
Leucine Zipper-Bearing Kinase Is a Critical Regulator of Astrocyte Reactivity in the Adult Mammalian CNS
Abstract
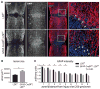
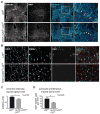
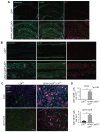
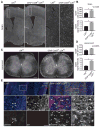
Reactive astrocytes influence post-injury recovery, repair, and pathogenesis of the mammalian CNS. Much of the regulation of astrocyte reactivity, however, remains to be understood. Using genetic loss and gain-of-function analyses in vivo, we show that the conserved MAP3K13 (also known as leucine zipper-bearing kinase [LZK]) promotes astrocyte reactivity and glial scar formation after CNS injury. Inducible LZK gene deletion in astrocytes of adult mice reduced astrogliosis and impaired glial scar formation, resulting in increased lesion size after spinal cord injury. Conversely, LZK overexpression in astrocytes enhanced astrogliosis and reduced lesion size. Remarkably, in the absence of injury, LZK overexpression alone induced widespread astrogliosis in the CNS and upregulated astrogliosis activators pSTAT3 and SOX9. The identification of LZK as a critical cell-intrinsic regulator of astrocyte reactivity expands our understanding of the multicellular response to CNS injury and disease, with broad translational implications for neural repair.
Keywords: CNS injury; LZK; MAP3K; SOX9; STAT3; astroglial reactivity; astrogliosis; glial scar; reactive astrocytes; spinal cord injury.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ben Haim L, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci. 2017;18:31–41. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

