Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study
- PMID: 29592805
- PMCID: PMC5875008
- DOI: 10.1186/s12916-018-1033-7
Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study
Abstract
Background: Drug-induced liver injury (DILI) is a common complication of tuberculosis treatment. We utilised data from the REMoxTB clinical trial to describe the incidence of predisposing factors and the natural history in patients with liver enzyme levels elevated in response to tuberculosis treatment.
Methods: Patients received either standard tuberculosis treatment (2EHRZ/4HR), or a 4-month regimen in which moxifloxacin replaced either ethambutol (isoniazid arm, 2MHRZ/2MHR) or isoniazid (ethambutol arm, 2EMRZ/2MR). Hepatic enzymes were measured at 0, 2, 4, 8, 12 and 17 weeks and as clinically indicated during reported adverse events. Patients included were those receiving at least one dose of drug and with two or more hepatic enzyme measurements.
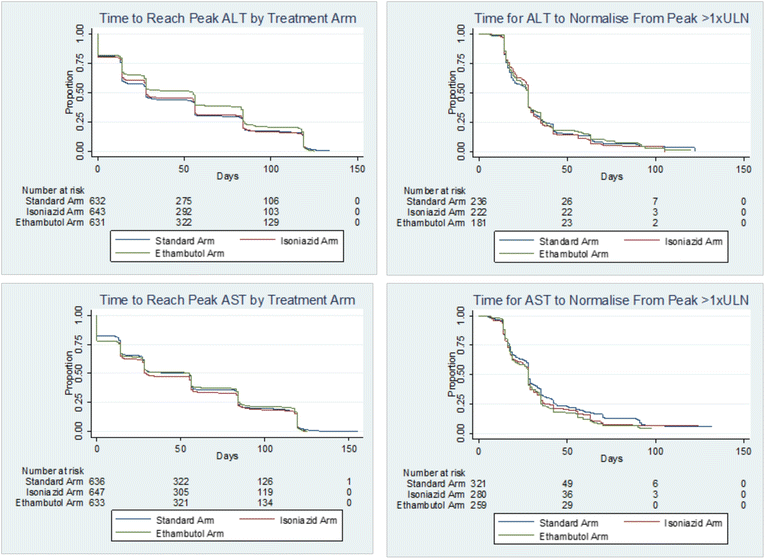
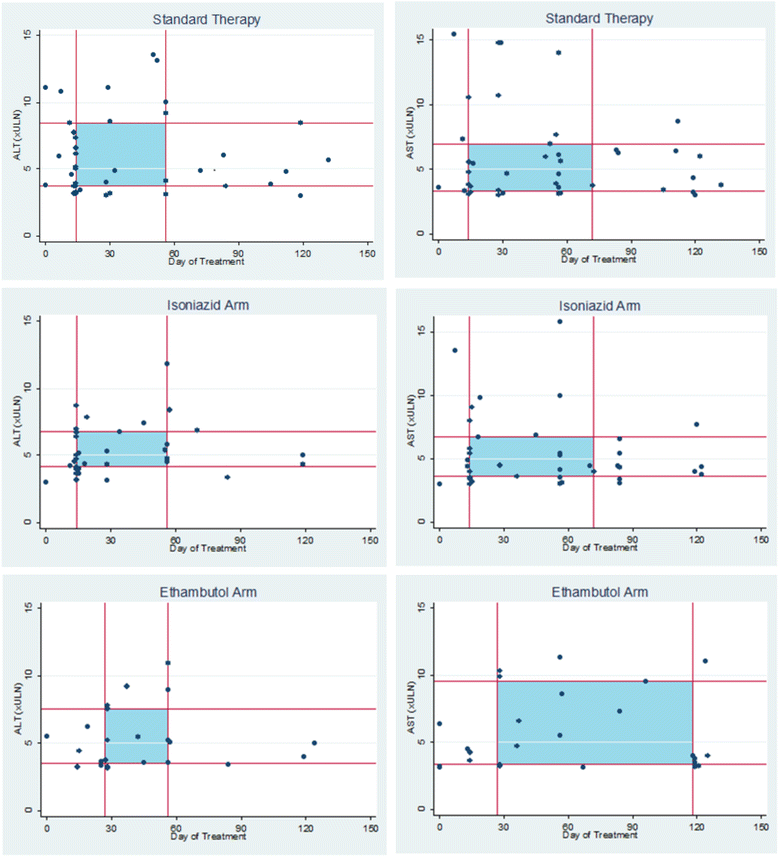
Results: A total of 1928 patients were included (639 2EHRZ/4HR, 654 2MHRZ/2MHR and 635 2EMRZ/2MR). DILI was defined as peak alanine aminotransferase (ALT) ≥ 5 times the upper limit of normal (5 × ULN) or ALT ≥ 3 × ULN with total bilirubin > 2 × ULN. DILI was identified in 58 of the 1928 (3.0%) patients at a median time of 28 days (interquartile range IQR 14-56). Of 639 (6.4%) patients taking standard tuberculosis therapy, 41 experienced clinically significant enzyme elevations (peak ALT ≥ 3 × ULN). On standard therapy, 21.1% of patients aged >55 years developed a peak ALT/aspartate aminotransferase (AST) ≥ 3 × ULN (p = 0.01) and 15% of HIV-positive patients experienced a peak ALT/AST ≥ 3 × ULN compared to 9% of HIV-negative patients (p = 0.160). The median peak ALT/AST was higher in isoniazid-containing regimens vs no-isoniazid regimens (p < 0.05), and lower in moxifloxacin-containing arms vs no-moxifloxacin arms (p < 0.05). Patients receiving isoniazid reached a peak ALT ≥ 3 × ULN 9.5 days earlier than those on the ethambutol arm (median time of 28 days vs 18.5 days). Of the 67 Asian patients with a peak ALT/AST ≥ 3 × ULN, 57 (85.1%) were on an isoniazid-containing regimen (p = 0.008).
Conclusions: Our results provide evidence of the risk of DILI in tuberculosis patients on standard treatment. Older patients on standard therapy, HIV-positive patients, Asian patients and those receiving isoniazid were at higher risk of elevated enzyme levels. Monitoring hepatic enzymes during the first 2 months of standard therapy detected approximately 75% of patients with a peak enzyme elevation ≥3 × ULN, suggesting this should be a standard of care. These results provide evidence for the potential of moxifloxacin in hepatic sparing.
Keywords: Drug-induced liver injury; Hepatotoxicity; Treatment monitoring; Tuberculosis.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was granted for REMoxTB by the ethics board at University College London, and at each of the study sites:
Kenya
Kenya Medical Research Institute (KEMRI) Scientific Steering Committee
KEMRI Ethical Review Committee
South Africa
Medicines Control Council, Pretoria
Pharma Ethics, Pretoria
University of Cape Town Human Research Ethics Committee, Cape Town
Biomedical Research Ethics Committee, Durban
Wits Human Research Ethics Committee, Johannesburg
Tanzania
Kilimanjaro Christian Medical College Research Ethics and Review Committee, Moshi
Mbeya Ethics and Research Committee, Mbeya
National Institute for Medical Research, Dar es Salaam
Zambia
University of Zambia Biomedical Research Ethics Committee, Lusaka
China
Beijing Chest Hospital of Capital Medical University Ethics Committee, Beijing
Shanghai Pulmonary Hospital Ethics Committee, Shanghai
Tianjin CDC Biomedical Ethics Committee, Tianjin
India
Biomedical Ethics Committee, New Delhi
Institutional Ethics Committee, Mahatma Gandhi Medical College and Hospital, Jaipur
Mexico
Comité de Investigación y Ética (División de Enseñanza, Investigación, Capacitación, Ética y Calidad) Hospital General de Occidente, Jalisco
National Jewish Health, Denver, USA
Thailand
Ethics Committees on Researches Involving Human Subjects, Rajavithi Hospital, Bangkok
The Khon Kaen University Ethics Committee for Human Research Faculty of Medicine, Khon Kaen University, Muang Khon Kaen
Ethical Review Committee for Research in Human Subjects, Ministry of Public Health, Nonthraburi
Ethical Review Committee of Chest Disease Institute, Department of Medical Services and Ministry of Public Health, Nonthraburi
Malaysia
Medical Research & Ethics Committee, Ministry of Health Malaysia, Kuala Lumpur
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council Tuberculosis Units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3:S231–S279. - PubMed
-
- Devarbhavi H. Antituberculosis drug-induced liver injury: current perspective. Trop Gastroenterol. 2011;32:167–174. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

