β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway
- PMID: 29592806
- PMCID: PMC5875012
- DOI: 10.1186/s12929-018-0431-7
β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway
Abstract
Background: β-aminoisobutyric acid (BAIBA) is produced in skeletal muscle during exercise and has beneficial effects on obesity-related metabolic disorders such as diabetes and non-alcoholic fatty liver disease. Thus, it is supposed to prevent high fat diet (HFD)-induced inflammation and insulin resistance in adipose tissue though anti-inflammatory effects in obesity. Previous reports have also demonstrated strong anti-inflammatory effects of BAIBA.
Methods: We used BAIBA treated fully differentiated 3T3T-L1 mouse adipocytes to investigate the effects of exogenous BAIBA on inflammation and insulin signaling in adipocytes. Insulin signaling-mediated proteins and inflammation markers were measured by Western blot analysis. Secretion of pro-inflammatory cytokines were measured by ELISA. Lipid accumulation in differentiated 3 T3-L1 cells was stained by Oil red-O. Statistical analysis was performed by ANOVA and student's t test.
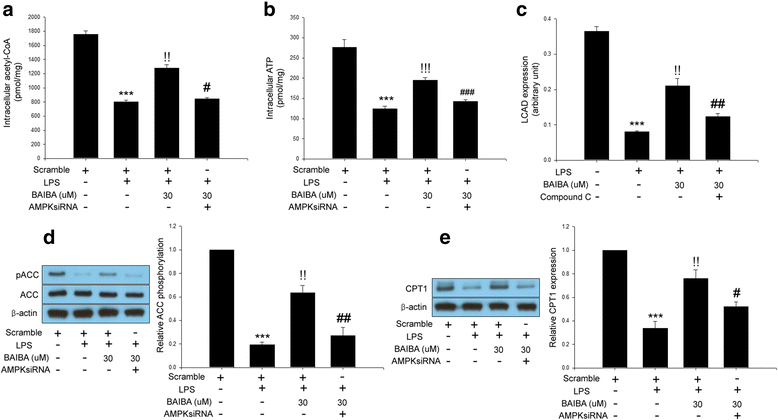
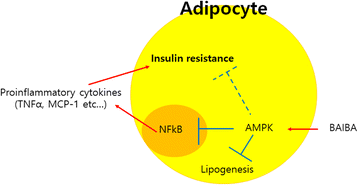
Results: BAIBA treatment suppressed adipogenesis assessed by adipogenic markers as well as lipid accumulation after full differentiation. We showed that BAIBA treatment stimulated AMP-activated protein kinase (AMPK) phosphorylation in a dose-dependent manner and lipopolysaccharide (LPS)-induced secretion of pro-inflammatory cytokines such as TNFα and MCP-1 was abrogated in BAIBA-treated 3 T3-L1 cells. Treatment of 3 T3-L1 cells with BAIBA reduced LPS-induced NFκB and IκB phosphorylation. Furthermore, BAIBA treatment ameliorated LPS-induced impairment of insulin signaling measured by IRS-1 and Akt phosphorylation and fatty acid oxidation. Suppression of AMPK by small interfering (si) RNA significantly restored these changes.
Conclusions: We demonstrated anti-inflammatory and anti-insulin resistance effects of BAIBA in differentiated 3 T3-L1 cells treated with LPS through AMPK-dependent signaling. These results provide evidence for the beneficial effects of BAIBA not only in liver and skeletal muscle cells but also in adipose tissue.
Keywords: AMPK; Adipocyte; BAIBA; Inflammation; Insulin resistance; NFκB.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in this work were in accordance with the ethical standards of the institutional and/or national research committee.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARδ-dependent pathway in mice.Diabetologia. 2015 Sep;58(9):2096-105. doi: 10.1007/s00125-015-3663-z. Epub 2015 Jun 24. Diabetologia. 2015. PMID: 26105792
-
BAIBA Attenuates the Expression of Inflammatory Cytokines and Attachment Molecules and ER Stress in HUVECs and THP-1 Cells.Pathobiology. 2018;85(5-6):280-288. doi: 10.1159/000490497. Epub 2018 Aug 3. Pathobiology. 2018. PMID: 30078017
-
β-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes.Sci Rep. 2016 Feb 24;6:21924. doi: 10.1038/srep21924. Sci Rep. 2016. PMID: 26907958 Free PMC article.
-
Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism.Nutrients. 2019 Feb 28;11(3):524. doi: 10.3390/nu11030524. Nutrients. 2019. PMID: 30823446 Free PMC article. Review.
-
Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation.Clin Sci (Lond). 2013 Apr;124(8):491-507. doi: 10.1042/CS20120536. Clin Sci (Lond). 2013. PMID: 23298225 Review.
Cited by
-
Identification of circulating metabolites associated with wooden breast and white striping.PLoS One. 2022 Sep 26;17(9):e0274208. doi: 10.1371/journal.pone.0274208. eCollection 2022. PLoS One. 2022. PMID: 36156596 Free PMC article.
-
Astragalus membranaceus Injection Suppresses Production of Interleukin-6 by Activating Autophagy through the AMPK-mTOR Pathway in Lipopolysaccharide-Stimulated Macrophages.Oxid Med Cell Longev. 2020 Jul 4;2020:1364147. doi: 10.1155/2020/1364147. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32724488 Free PMC article.
-
The function of previously unappreciated exerkines secreted by muscle in regulation of neurodegenerative diseases.Front Mol Neurosci. 2024 Jan 5;16:1305208. doi: 10.3389/fnmol.2023.1305208. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38249295 Free PMC article. Review.
-
Beta-Aminoisobutyric Acid Inhibits Hypothalamic Inflammation by Reversing Microglia Activation.Cells. 2019 Dec 11;8(12):1609. doi: 10.3390/cells8121609. Cells. 2019. PMID: 31835795 Free PMC article.
-
Enoxaparin sodium bone cement displays local anti-inflammatory effects by regulating the expression of IL-6 and TNF-α.Heliyon. 2023 May 25;9(6):e16530. doi: 10.1016/j.heliyon.2023.e16530. eCollection 2023 Jun. Heliyon. 2023. PMID: 37274684 Free PMC article.
References
-
- Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117(6):241–250. - PubMed
-
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–2971. - PubMed
-
- Begriche K, Massart J, Abbey-Toby A, Igoudjil A, Letteron P, Fromenty B. Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity. 2008;16(9):2053–2067. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

