The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death
- PMID: 29592816
- PMCID: PMC5875007
- DOI: 10.1186/s12974-018-1134-4
The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death
Abstract
Background: Currently, several amyloid beta (Aβ) antibodies, including the protofibril selective antibody BAN2401, are in clinical trials. The murine version of BAN2401, mAb158, has previously been shown to lower the levels of pathogenic Aβ and prevent Aβ deposition in animal models of Alzheimer's disease (AD). However, the cellular mechanisms of the antibody's action remain unknown. We have recently shown that astrocytes effectively engulf Aβ42 protofibrils, but store rather than degrade the ingested Aβ aggregates. In a co-culture set-up, the incomplete degradation of Aβ42 protofibrils by astrocytes results in increased neuronal cell death, due to the release of extracellular vesicles, containing N-truncated, neurotoxic Aβ.
Methods: The aim of the present study was to investigate if the accumulation of Aβ in astrocytes can be affected by the Aβ protofibril selective antibody mAb158. Co-cultures of astrocytes, neurons, and oligodendrocytes, derived from embryonic mouse cortex, were exposed to Aβ42 protofibrils in the presence or absence of mAb158.
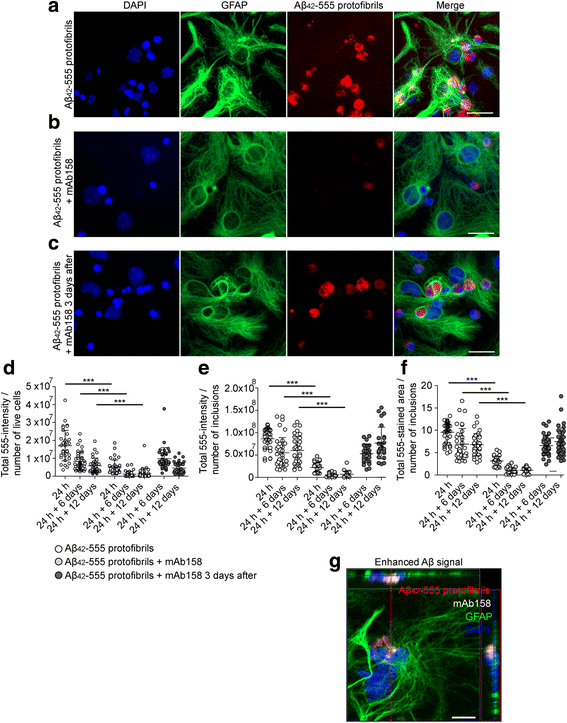
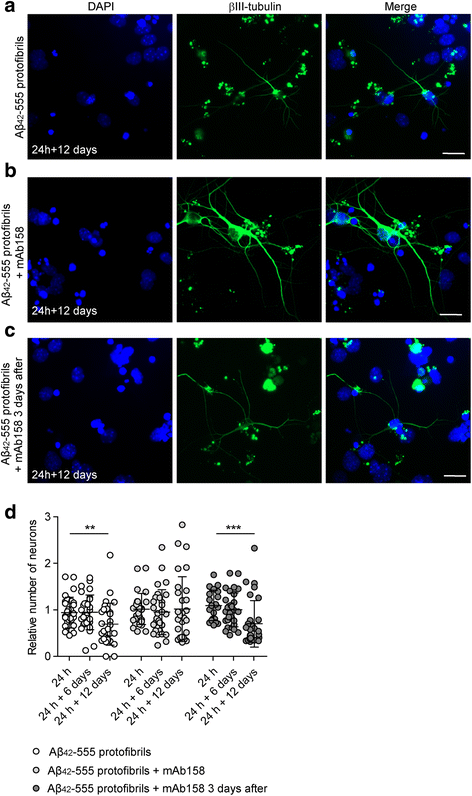
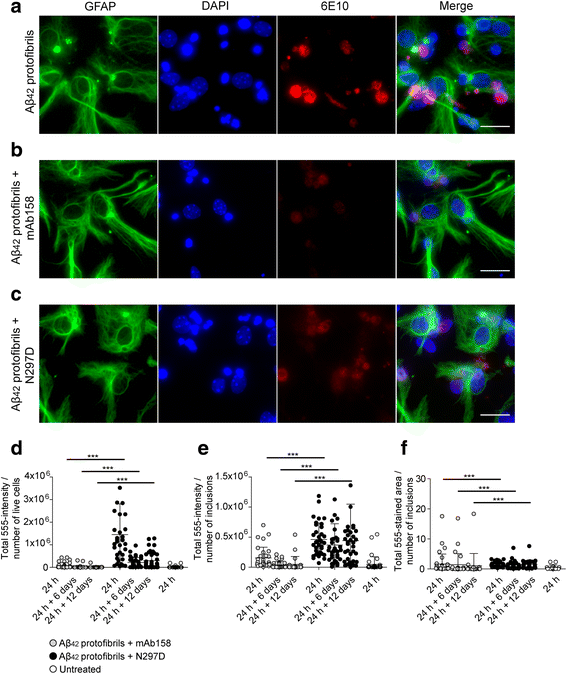
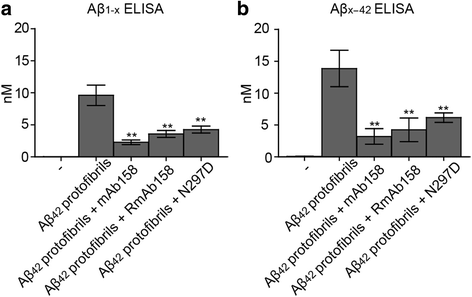
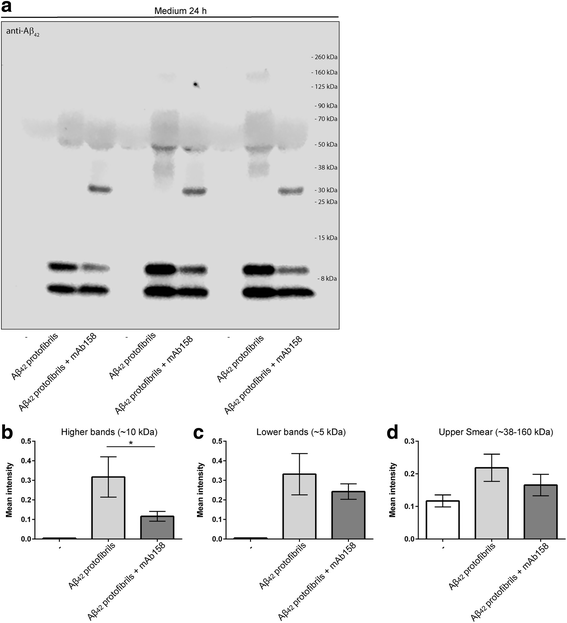
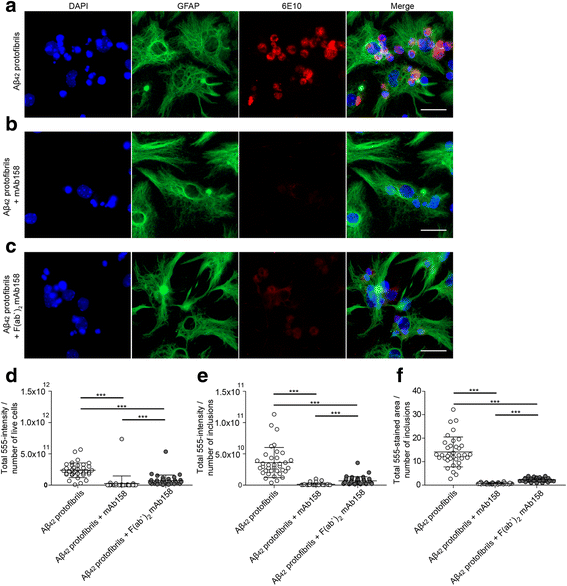
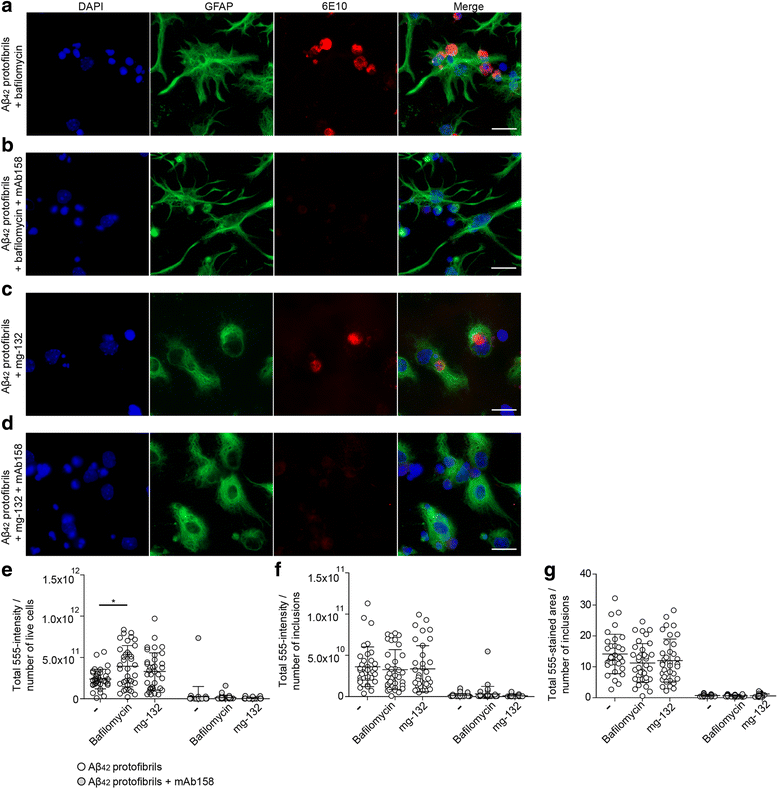
Results: Our results demonstrate that the presence of mAb158 almost abolished Aβ accumulation in astrocytes. Consequently, mAb158 treatment rescued neurons from Aβ-induced cell death.
Conclusion: Based on these findings, we conclude that astrocytes may play a central mechanistic role in anti-Aβ immunotherapy.
Keywords: Alzheimer’s disease; Amyloid-β; Antibody; Astrocyte; Clearance; Neuron.
Conflict of interest statement
Ethics approval and consent to participate
All experiments involving animals were performed at Uppsala University, Sweden. The experiments were approved by the Uppsala County Animal Ethics Board (ethical permit number: C75/13, valid 2013-06-28 to 2018-06-28), following the rules and regulations of the Swedish Animal Welfare Agency, in compliance with the European Communities Council Directive of 22 September 2010 (2010/63/EU).
Consent for publication
Not applicable.
Competing interests
LS is employed by BioArctic AB. LL is a co-founder of BioArctic AB and stock owner. This does not alter to the
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice.J Alzheimers Dis. 2015;43(2):575-88. doi: 10.3233/JAD-140741. J Alzheimers Dis. 2015. PMID: 25096615
-
Disease modifying effects of the amyloid-beta protofibril-selective antibody mAb158 in aged Tg2576 transgenic mice.Mol Cell Neurosci. 2024 Sep;130:103950. doi: 10.1016/j.mcn.2024.103950. Epub 2024 Jun 18. Mol Cell Neurosci. 2024. PMID: 38901655
-
Accumulation of amyloid-β by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons.Mol Neurodegener. 2016 May 12;11(1):38. doi: 10.1186/s13024-016-0098-z. Mol Neurodegener. 2016. PMID: 27176225 Free PMC article.
-
Perspectives on future Alzheimer therapies: amyloid-β protofibrils - a new target for immunotherapy with BAN2401 in Alzheimer's disease.Alzheimers Res Ther. 2014 Mar 24;6(2):16. doi: 10.1186/alzrt246. eCollection 2014. Alzheimers Res Ther. 2014. PMID: 25031633 Free PMC article. Review.
-
Calcium-Sensing Receptors of Human Neural Cells Play Crucial Roles in Alzheimer's Disease.Front Physiol. 2016 Apr 26;7:134. doi: 10.3389/fphys.2016.00134. eCollection 2016. Front Physiol. 2016. PMID: 27199760 Free PMC article. Review.
Cited by
-
The Neurovascular Unit Dysfunction in Alzheimer's Disease.Int J Mol Sci. 2021 Feb 18;22(4):2022. doi: 10.3390/ijms22042022. Int J Mol Sci. 2021. PMID: 33670754 Free PMC article. Review.
-
Neuroprotective Approach of Anti-Cancer Microtubule Stabilizers Against Tauopathy Associated Dementia: Current Status of Clinical and Preclinical Findings.J Alzheimers Dis Rep. 2019 Jul 2;3(1):179-218. doi: 10.3233/ADR-190125. J Alzheimers Dis Rep. 2019. PMID: 31435618 Free PMC article. Review.
-
Alzheimer's Disease: A Brief History of Immunotherapies Targeting Amyloid β.Int J Mol Sci. 2023 Feb 15;24(4):3895. doi: 10.3390/ijms24043895. Int J Mol Sci. 2023. PMID: 36835301 Free PMC article. Review.
-
Photobiomodulation in Alzheimer's Disease-A Complementary Method to State-of-the-Art Pharmaceutical Formulations and Nanomedicine?Pharmaceutics. 2023 Mar 11;15(3):916. doi: 10.3390/pharmaceutics15030916. Pharmaceutics. 2023. PMID: 36986776 Free PMC article. Review.
-
Therapeutic Strategies to Reduce the Toxicity of Misfolded Protein Oligomers.Int J Mol Sci. 2020 Nov 17;21(22):8651. doi: 10.3390/ijms21228651. Int J Mol Sci. 2020. PMID: 33212787 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

