Selectins and chemokines use shared and distinct signals to activate β2 integrins in neutrophils
- PMID: 29592875
- PMCID: PMC5894262
- DOI: 10.1182/bloodadvances.2017015602
Selectins and chemokines use shared and distinct signals to activate β2 integrins in neutrophils
Abstract
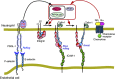
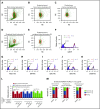
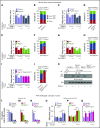
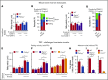
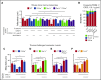
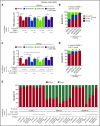
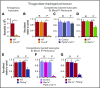
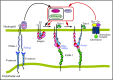
Rolling neutrophils receive signals while engaging P- and E-selectin and chemokines on inflamed endothelium. Selectin signaling activates β2 integrins to slow rolling velocities. Chemokine signaling activates β2 integrins to cause arrest. Despite extensive study, key aspects of these signaling cascades remain unresolved. Using complementary in vitro and in vivo assays, we found that selectin and chemokine signals in neutrophils triggered Rap1a-dependent and phosphatidylinositol-4-phosphate 5-kinase γ (PIP5Kγ90)-dependent pathways that induce integrin-dependent slow rolling and arrest. Interruption of both pathways, but not either pathway alone, blocked talin-1 recruitment to and activation of integrins. An isoform of PIP5Kγ90 lacking the talin-binding domain (PIP5Kγ87) could not activate integrins. Chemokines, but not selectins, used phosphatidylinositol-4,5-bisphosphate 3-kinase γ (PI3Kγ) in cooperation with Rap1a to mediate integrin-dependent slow rolling (at low chemokine concentrations), as well as arrest (at high chemokine concentrations). High levels of chemokines activated β2 integrins without selectin signals. When chemokines were limiting, they synergized with selectins to activate β2 integrins.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.P.M. is a cofounder of Selexys Pharmaceuticals, now part of Novartis AG, and of Tetherex Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling.Eur J Immunol. 2011 Jul;41(7):2074-85. doi: 10.1002/eji.201041196. Epub 2011 Jun 7. Eur J Immunol. 2011. PMID: 21480213 Free PMC article.
-
Structural Basis of β2 Integrin Inside-Out Activation.Cells. 2022 Sep 28;11(19):3039. doi: 10.3390/cells11193039. Cells. 2022. PMID: 36231001 Free PMC article. Review.
-
Binding of Rap1 and Riam to Talin1 Fine-Tune β2 Integrin Activity During Leukocyte Trafficking.Front Immunol. 2021 Aug 19;12:702345. doi: 10.3389/fimmu.2021.702345. eCollection 2021. Front Immunol. 2021. PMID: 34489950 Free PMC article.
-
Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways.Blood. 2010 Apr 15;115(15):3118-27. doi: 10.1182/blood-2009-11-254185. Epub 2010 Feb 18. Blood. 2010. PMID: 20167705 Free PMC article.
-
Selectins: initiators of leucocyte adhesion and signalling at the vascular wall.Cardiovasc Res. 2015 Aug 1;107(3):331-9. doi: 10.1093/cvr/cvv154. Epub 2015 May 20. Cardiovasc Res. 2015. PMID: 25994174 Free PMC article. Review.
Cited by
-
Neutrophils-biology and diversity.Nephrol Dial Transplant. 2024 Sep 27;39(10):1551-1564. doi: 10.1093/ndt/gfad266. Nephrol Dial Transplant. 2024. PMID: 38115607 Free PMC article. Review.
-
Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment.Front Immunol. 2019 May 22;10:1078. doi: 10.3389/fimmu.2019.01078. eCollection 2019. Front Immunol. 2019. PMID: 31231358 Free PMC article. Review.
-
Nanoscopy reveals integrin clustering reliant on kindlin-3 but not talin-1.Cell Commun Signal. 2025 Jan 7;23(1):12. doi: 10.1186/s12964-024-02024-8. Cell Commun Signal. 2025. PMID: 39773732 Free PMC article.
-
Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis.Acta Pharm Sin B. 2022 Jan;12(1):18-32. doi: 10.1016/j.apsb.2021.07.023. Epub 2021 Jul 29. Acta Pharm Sin B. 2022. PMID: 35127370 Free PMC article. Review.
-
Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice.Blood. 2018 Sep 27;132(13):1426-1437. doi: 10.1182/blood-2018-05-850859. Epub 2018 Aug 1. Blood. 2018. PMID: 30068506 Free PMC article.
References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678-689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

