Warfarin alters vitamin K metabolism: a surprising mechanism of VKORC1 uncoupling necessitates an additional reductase
- PMID: 29592891
- PMCID: PMC6014353
- DOI: 10.1182/blood-2017-09-804666
Warfarin alters vitamin K metabolism: a surprising mechanism of VKORC1 uncoupling necessitates an additional reductase
Abstract
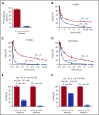
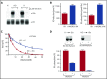
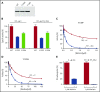
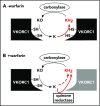
The anticoagulant warfarin inhibits the vitamin K oxidoreductase (VKORC1), which generates vitamin K hydroquinone (KH2) required for the carboxylation and consequent activation of vitamin K-dependent (VKD) proteins. VKORC1 produces KH2 in 2 reactions: reduction of vitamin K epoxide (KO) to quinone (K), and then KH2 Our dissection of full reduction vs the individual reactions revealed a surprising mechanism of warfarin inhibition. Warfarin inhibition of KO to K reduction and carboxylation that requires full reduction were compared in wild-type VKORC1 or mutants (Y139H, Y139F) that cause warfarin resistance. Carboxylation was much more strongly inhibited (∼400-fold) than KO reduction (two- to threefold). The K to KH2 reaction was analyzed using low K concentrations that result from inhibition of KO to K. Carboxylation that required only K to KH2 reduction was inhibited much less than observed with the KO substrate that requires full VKORC1 reduction (eg, 2.5-fold vs 70-fold, respectively, in cells expressing wild-type VKORC1 and factor IX). The results indicate that warfarin uncouples the 2 reactions that fully reduce KO. Uncoupling was revealed because a second activity, a warfarin-resistant quinone reductase, was not present. In contrast, 293 cells expressing factor IX and this reductase activity showed much less inhibition of carboxylation. This activity therefore appears to cooperate with VKORC1 to accomplish full KO reduction. Cooperation during warfarin therapy would have significant consequences, as VKD proteins function in numerous physiologies in many tissues, but may be poorly carboxylated and dysfunctional if the second activity is not ubiquitously expressed similar to VKORC1.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




Comment in
-
Warfarin, a juggler's demise.Blood. 2018 Jun 21;131(25):2742-2743. doi: 10.1182/blood-2018-05-843151. Blood. 2018. PMID: 29930151 Free PMC article.
Similar articles
-
The vitamin K oxidoreductase is a multimer that efficiently reduces vitamin K epoxide to hydroquinone to allow vitamin K-dependent protein carboxylation.J Biol Chem. 2013 Nov 1;288(44):31556-66. doi: 10.1074/jbc.M113.497297. Epub 2013 Aug 5. J Biol Chem. 2013. PMID: 23918929 Free PMC article.
-
VKORC1L1, An Enzyme Mediating the Effect of Vitamin K in Liver and Extrahepatic Tissues.Nutrients. 2018 Jul 26;10(8):970. doi: 10.3390/nu10080970. Nutrients. 2018. PMID: 30050002 Free PMC article. Review.
-
Stabilization of warfarin-binding pocket of VKORC1 and VKORL1 by a peripheral region determines their different sensitivity to warfarin inhibition.J Thromb Haemost. 2018 Jun;16(6):1164-1175. doi: 10.1111/jth.14127. Epub 2018 May 20. J Thromb Haemost. 2018. PMID: 29665197 Free PMC article.
-
Functional study of the vitamin K cycle in mammalian cells.Blood. 2011 Mar 10;117(10):2967-74. doi: 10.1182/blood-2010-08-304303. Epub 2011 Jan 14. Blood. 2011. PMID: 21239697 Free PMC article.
-
[Vitamin K epoxide reductase: Fresh blood for oral anticoagulant therapies].Rev Med Interne. 2006 Dec;27(12):979-82. doi: 10.1016/j.revmed.2006.09.004. Epub 2006 Oct 11. Rev Med Interne. 2006. PMID: 17070618 Review. French.
Cited by
-
Frequency of prothrombin time-international normalized ratio monitoring and the long-term prognosis in patients with mechanical valve replacement.BMC Cardiovasc Disord. 2023 Jun 24;23(1):322. doi: 10.1186/s12872-023-03293-w. BMC Cardiovasc Disord. 2023. PMID: 37355558 Free PMC article.
-
A Chinese patient with cardiogenic stroke and warfarin resistance: a case report.BMC Neurol. 2025 Feb 24;25(1):77. doi: 10.1186/s12883-025-04088-6. BMC Neurol. 2025. PMID: 39994532 Free PMC article.
-
Drug-microbiota interactions: an emerging priority for precision medicine.Signal Transduct Target Ther. 2023 Oct 9;8(1):386. doi: 10.1038/s41392-023-01619-w. Signal Transduct Target Ther. 2023. PMID: 37806986 Free PMC article. Review.
-
Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis.J Clin Med. 2021 May 29;10(11):2419. doi: 10.3390/jcm10112419. J Clin Med. 2021. PMID: 34072487 Free PMC article. Review.
-
GGCX mutants that impair hemostasis reveal the importance of processivity and full carboxylation to VKD protein function.Blood. 2022 Oct 13;140(15):1710-1722. doi: 10.1182/blood.2021014275. Blood. 2022. PMID: 35767717 Free PMC article.
References
-
- Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131-156. - PubMed
-
- Oldenburg J, Müller CR, Rost S, Watzka M, Bevans CG. Comparative genetics of warfarin resistance. Hamostaseologie. 2014;34(2):143-159. - PubMed
-
- Watzka M, Geisen C, Scheer M, et al. . Bleeding and non-bleeding phenotypes in patients with GGCX gene mutations. Thromb Res. 2014;134(4):856-865. - PubMed
-
- Spohn G, Kleinridders A, Wunderlich FT, et al. . VKORC1 deficiency in mice causes early postnatal lethality due to severe bleeding. Thromb Haemost. 2009;101(6):1044-1050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

