Single Degranulations in NK Cells Can Mediate Target Cell Killing
- PMID: 29592963
- PMCID: PMC6020067
- DOI: 10.4049/jimmunol.1701500
Single Degranulations in NK Cells Can Mediate Target Cell Killing
Abstract
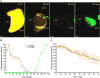
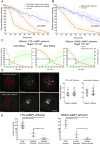
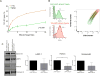
NK cells are cytotoxic lymphocytes important in defense against viral infection and cancer. NK cells mediate cytotoxicity predominantly through directed secretion of lytic granules, which are specialized lysosome-related organelles, containing effector molecules such as perforin and granzymes. Although many requirements for lytic granule transport to, and secretion at, the NK cell lytic synapse are known, the minimum number of degranulation events required by an NK cell to kill its target is unknown. We performed high-resolution four-dimensional confocal microscopy of human NK-target cell conjugates to quantify NK cell degranulation (using a degranulation indicator, LAMP-1-pHluorin) as well as target cell death. Despite containing almost 200 granules, we found that an individual NK cell needed only two to four degranulation events, on average, to mediate target cell death. Although NK cells released approximately one-tenth of their total lytic granule reserve upon a single target, they required just over one-hundredth of their total lytic granules to kill a target cell. Importantly, the kinetics of NK cell killing correlated to the size of and the amount of effector molecules contained within lytic granules, as well as the temporal, but not spatial, organization of degranulation events. Thus, our study answers a fundamental question as to how many degranulation events it takes for a human NK cell to kill its target.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures



References
-
- Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

