T-cell Dysfunction in Glioblastoma: Applying a New Framework
- PMID: 29593027
- PMCID: PMC6095741
- DOI: 10.1158/1078-0432.CCR-18-0047
T-cell Dysfunction in Glioblastoma: Applying a New Framework
Abstract
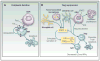
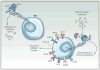
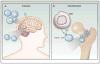
A functional, replete T-cell repertoire is an integral component to adequate immune surveillance and to the initiation and maintenance of productive antitumor immune responses. Glioblastoma (GBM), however, is particularly adept at sabotaging antitumor immunity, eliciting severe T-cell dysfunction that is both qualitative and quantitative. Understanding and countering such dysfunction are among the keys to harnessing the otherwise stark potential of anticancer immune-based therapies. Although T-cell dysfunction in GBM has been long described, newer immunologic frameworks now exist for reclassifying T-cell deficits in a manner that better permits their study and reversal. Herein, we divide and discuss the various T-cell deficits elicited by GBM within the context of the five relevant categories: senescence, tolerance, anergy, exhaustion, and ignorance. Categorization is appropriately made according to the molecular bases of dysfunction. Likewise, we review the mechanisms by which GBM elicits each mode of T-cell dysfunction and discuss the emerging immunotherapeutic strategies designed to overcome them. Clin Cancer Res; 24(16); 3792-802. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Erlich P. Über den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273–90.
-
- Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery. 2012;71:201–22. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Brooks WH, Horwitz DA, Netsky MG. Evidence for tumor-specific immune response in patients with primary brain tumors. Surg Forum. 1972;23:430–2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

