Extracellular Conformational Changes in the Capsid of Human Papillomaviruses Contribute to Asynchronous Uptake into Host Cells
- PMID: 29593032
- PMCID: PMC5952151
- DOI: 10.1128/JVI.02106-17
Extracellular Conformational Changes in the Capsid of Human Papillomaviruses Contribute to Asynchronous Uptake into Host Cells
Abstract
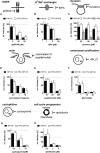
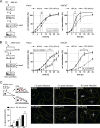
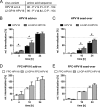
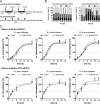
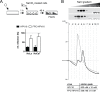
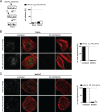
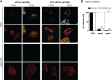
Human papillomavirus 16 (HPV16) is the leading cause of cervical cancer. For initial infection, HPV16 utilizes a novel endocytic pathway for host cell entry. Unique among viruses, uptake occurs asynchronously over a protracted period of time, with half-times between 9 and 12 h. To trigger endocytic uptake, the virus particles need to undergo a series of structural modifications after initial binding to heparan sulfate proteoglycans (HSPGs). These changes involve proteolytic cleavage of the major capsid protein L1 by kallikrein-8 (KLK8), exposure of the N terminus of the minor capsid protein L2 by cyclophilins, and cleavage of this N terminus by furin. Overall, the structural changes are thought to facilitate the engagement of an elusive secondary receptor for internalization. Here, we addressed whether structural changes are the rate-limiting steps during infectious internalization of HPV16 by using structurally primed HPV16 particles. Our findings indicate that the structural modifications mediated by cyclophilins and furin, which lead to exposure and cleavage, respectively, of the L2 N terminus contribute to the slow and asynchronous internalization kinetics, whereas conformational changes elicited by HSPG binding and KLK8 cleavage did not. However, these structural modifications accounted for only 30 to 50% of the delay in internalization. Therefore, we propose that limited internalization receptor availability for engagement of HPV16 causes slow and asynchronous internalization in addition to rate-limiting structural changes in the viral capsid.IMPORTANCE HPVs are the main cause of anogenital cancers. Their unique biology is linked to the differentiation program of skin or mucosa. Here, we analyzed another unique aspect of HPV infections using the prototype HPV16. After initial cell binding, HPVs display an unusually protracted residence time on the plasma membrane prior to asynchronous uptake. As viruses typically do not expose themselves to host immune sensing, we analyzed the underlying reasons for this unusual behavior. This study provides evidence that both extracellular structural modifications and possibly a limited availability of the internalization receptor contribute to the slow internalization process of the virus. These findings indicate that perhaps a unique niche for initial infection that could allow for rapid infection exists. In addition, our results may help to develop novel, preventive antiviral measures.
Keywords: furin; kinetic; papillomavirus; structural modification; virus entry.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
Kallikrein-8 Proteolytically Processes Human Papillomaviruses in the Extracellular Space To Facilitate Entry into Host Cells.J Virol. 2015 Jul;89(14):7038-52. doi: 10.1128/JVI.00234-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926655 Free PMC article.
-
Vimentin Modulates Infectious Internalization of Human Papillomavirus 16 Pseudovirions.J Virol. 2017 Jul 27;91(16):e00307-17. doi: 10.1128/JVI.00307-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28566373 Free PMC article.
-
Epidermal Growth Factor Receptor and Abl2 Kinase Regulate Distinct Steps of Human Papillomavirus 16 Endocytosis.J Virol. 2020 May 18;94(11):e02143-19. doi: 10.1128/JVI.02143-19. Print 2020 May 18. J Virol. 2020. PMID: 32188731 Free PMC article.
-
The evolving field of human papillomavirus receptor research: a review of binding and entry.J Virol. 2013 Jun;87(11):6062-72. doi: 10.1128/JVI.00330-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536685 Free PMC article. Review.
-
Mechanisms of cell entry by human papillomaviruses: an overview.Virol J. 2010 Jan 20;7:11. doi: 10.1186/1743-422X-7-11. Virol J. 2010. PMID: 20089191 Free PMC article. Review.
Cited by
-
Master mitotic kinases regulate viral genome delivery during papillomavirus cell entry.Nat Commun. 2023 Jan 23;14(1):355. doi: 10.1038/s41467-023-35874-w. Nat Commun. 2023. PMID: 36683055 Free PMC article.
-
Diversity in Proprotein Convertase Reactivity among Human Papillomavirus Types.Viruses. 2023 Dec 26;16(1):39. doi: 10.3390/v16010039. Viruses. 2023. PMID: 38257739 Free PMC article.
-
Glycan-induced structural activation softens the human papillomavirus capsid for entry through reduction of intercapsomere flexibility.Nat Commun. 2024 Nov 21;15(1):10076. doi: 10.1038/s41467-024-54373-0. Nat Commun. 2024. PMID: 39572555 Free PMC article.
-
A Ran-binding protein facilitates nuclear import of human papillomavirus type 16.PLoS Pathog. 2021 May 11;17(5):e1009580. doi: 10.1371/journal.ppat.1009580. eCollection 2021 May. PLoS Pathog. 2021. PMID: 33974675 Free PMC article.
-
Heparan Sulfate and Sialic Acid in Viral Attachment: Two Sides of the Same Coin?Int J Mol Sci. 2022 Aug 30;23(17):9842. doi: 10.3390/ijms23179842. Int J Mol Sci. 2022. PMID: 36077240 Free PMC article. Review.
References
-
- Klug A, Finch JT. 1965. Structure of viruses of the papilloma-polyoma type. I. Human wart virus. J Mol Biol 11:403–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

