Host Factor SPCS1 Regulates the Replication of Japanese Encephalitis Virus through Interactions with Transmembrane Domains of NS2B
- PMID: 29593046
- PMCID: PMC5974503
- DOI: 10.1128/JVI.00197-18
Host Factor SPCS1 Regulates the Replication of Japanese Encephalitis Virus through Interactions with Transmembrane Domains of NS2B
Abstract
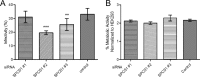
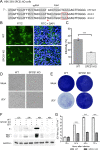
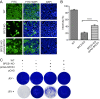
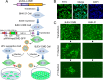
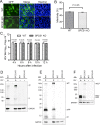
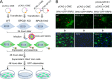
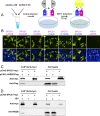
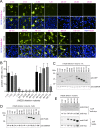
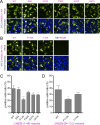
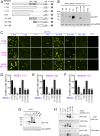
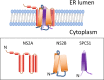
Signal peptidase complex subunit 1 (SPCS1) is a newly identified host factor that regulates flavivirus replication, but the molecular mechanism is not fully understood. Here, using Japanese encephalitis virus (JEV) as a model, we investigated the mechanism through which the host factor SPCS1 regulates the replication of flaviviruses. We first validated the regulatory function of SPCS1 in JEV propagation by knocking down and knocking out endogenous SPCS1. The loss of SPCS1 function markedly reduced intracellular virion assembly and the production of infectious JEV particles but did not affect cell entry, RNA replication, or translation of the virus. SPCS1 was found to interact with nonstructural protein 2B (NS2B), which is involved in posttranslational protein processing and virus assembly. Serial deletion mutation of the JEV NS2B protein revealed that two transmembrane domains, NS2B(1-49) and NS2B(84-131), interact with SPCS1. Further mutagenesis analysis of conserved flavivirus residues in two SPCS1 interaction domains of NS2B demonstrated that G12A, G37A, and G47A in NS2B(1-49) and P112A in NS2B(84-131) weakened the interaction with SPCS1. Deletion mutation of SPCS1 revealed that SPCS1(91-169), which contains two transmembrane domains, was involved in interactions with both NS2B(1-49) and NS2B(84-131). Taken together, these results demonstrate that SPCS1 affects viral replication by interacting with NS2B, thereby influencing the posttranslational processing of JEV proteins and the assembly of virions.IMPORTANCE Understanding virus-host interactions is important for elucidating the molecular mechanisms of virus propagation and identifying potential antiviral targets. Previous reports demonstrated that SPCS1 is involved in the flavivirus life cycle, but the mechanism remains unknown. In this study, we confirmed that SPCS1 participates in the posttranslational protein processing and viral assembly stages of the JEV life cycle but not in the cell entry, genome RNA replication, or translation stages. Furthermore, we found that SPCS1 interacts with two independent transmembrane domains of the flavivirus NS2B protein. NS2B also interacts with NS2A, which is proposed to mediate virus assembly. Therefore, we propose a protein-protein interaction model showing how SPCS1 participates in the assembly of JEV particles. These findings expand our understanding of how host factors participate in the flavivirus replication life cycle and identify potential antiviral targets for combating flavivirus infection.
Keywords: assembly; flavivirus; host factor; protein-protein interactions; viral replication.
Copyright © 2018 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

