Effect of Short-Term Antiretroviral Therapy Interruption on Levels of Integrated HIV DNA
- PMID: 29593048
- PMCID: PMC5974505
- DOI: 10.1128/JVI.00285-18
Effect of Short-Term Antiretroviral Therapy Interruption on Levels of Integrated HIV DNA
Abstract
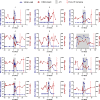
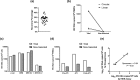
Analytic treatment interruption (ATI) studies are required to evaluate strategies aimed at achieving ART-free HIV remission, but the impact of ATI on the viral reservoir remains unclear. We validated a DNA size selection-based assay for measuring levels of integrated HIV DNA and applied it to assess the effects of short-term ATI on the HIV reservoir. Samples from participants from four AIDS Clinical Trials Group ATI studies were assayed for integrated HIV DNA levels. Cryopreserved peripheral blood mononuclear cells (PBMCs) were obtained for 12 participants with available samples pre-ATI and approximately 6 months after ART resumption. Four participants also had samples available during the ATI. The median duration of ATI was 12 weeks. Validation of the HIV integrated DNA size-exclusion (HIDE) assay was performed using samples spiked with unintegrated HIV DNA, HIV-infected cell lines, and participant PBMCs. The HIDE assay eliminated 99% of unintegrated HIV DNA species and strongly correlated with the established Alu-gag assay. For the majority of individuals, integrated DNA levels increased during ATI and subsequently declined upon ART resumption. There was no significant difference in the levels of integrated HIV DNA between the pre- and post-ATI time points, with a median ratio of post- to pre-ATI HIV DNA levels of 0.95. Using a new integrated HIV DNA assay, we found minimal change in the levels of integrated HIV DNA in participants who underwent an ATI, followed by 6 months of ART. This suggests that short-term ATI can be conducted without a significant impact on the levels of integrated proviral DNA in the peripheral blood.IMPORTANCE Interventions aimed at achieving sustained antiretroviral therapy (ART)-free HIV remission require treatment interruption trials to assess their efficacy. However, these trials are accompanied by safety concerns related to the expansion of the viral reservoir. We validated an assay that uses an automated DNA size-selection platform for quantifying levels of integrated HIV DNA and is less sample- and labor-intensive than current assays. Using stored samples from AIDS Clinical Trials Group studies, we found that short-term ART discontinuation had minimal impact on integrated HIV DNA levels after ART resumption, providing reassurance about the reservoir effects of short-term treatment interruption trials.
Keywords: HIV; assay; integrated DNA; reservoir; treatment interruption.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Impact of long-term antiretroviral therapy interruption and resumption on viral reservoir in HIV-1 infected patients.AIDS. 2017 Aug 24;31(13):1895-1897. doi: 10.1097/QAD.0000000000001560. AIDS. 2017. PMID: 28590333
-
The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption.AIDS. 2016 Jan 28;30(3):343-53. doi: 10.1097/QAD.0000000000000953. AIDS. 2016. PMID: 26588174 Free PMC article.
-
Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals.PLoS Pathog. 2018 Jan 11;14(1):e1006792. doi: 10.1371/journal.ppat.1006792. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29324842 Free PMC article.
-
Initiation of Antiretroviral Therapy during Primary HIV Infection: Effects on the Latent HIV Reservoir, Including on Analytic Treatment Interruptions.AIDS Rev. 2020 Oct 26;23(1):28-39. doi: 10.24875/AIDSRev.20000001. AIDS Rev. 2020. PMID: 33105471 Free PMC article.
-
Lessons learned from HIV antiretroviral treatment interruption trials.Curr Opin HIV AIDS. 2018 Sep;13(5):416-421. doi: 10.1097/COH.0000000000000484. Curr Opin HIV AIDS. 2018. PMID: 29878912 Review.
Cited by
-
Transient viral replication during analytical treatment interruptions in SIV infected macaques can alter the rebound-competent viral reservoir.PLoS Pathog. 2021 Jun 18;17(6):e1009686. doi: 10.1371/journal.ppat.1009686. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34143853 Free PMC article.
-
Ethical issues in HIV remission trials.Curr Opin HIV AIDS. 2018 Sep;13(5):422-427. doi: 10.1097/COH.0000000000000489. Curr Opin HIV AIDS. 2018. PMID: 30015634 Free PMC article. Review.
-
Dynamic Shifts in the HIV Proviral Landscape During Long Term Combination Antiretroviral Therapy: Implications for Persistence and Control of HIV Infections.Viruses. 2020 Jan 25;12(2):136. doi: 10.3390/v12020136. Viruses. 2020. PMID: 31991737 Free PMC article.
-
Insight into treatment of HIV infection from viral dynamics models.Immunol Rev. 2018 Sep;285(1):9-25. doi: 10.1111/imr.12698. Immunol Rev. 2018. PMID: 30129208 Free PMC article. Review.
-
Antiretroviral Therapy Interruption (ATI) in HIV-1 Infected Patients Participating in Therapeutic Vaccine Trials: Surrogate Markers of Virological Response.Vaccines (Basel). 2020 Aug 5;8(3):442. doi: 10.3390/vaccines8030442. Vaccines (Basel). 2020. PMID: 32764508 Free PMC article. Review.
References
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. doi:10.1038/8394. - DOI - PubMed
-
- Gandhi RT, McMahon DK, Bosch RJ, Lalama CM, Cyktor JC, Macatangay BJ, Rinaldo CR, Riddler SA, Hogg E, Godfrey C, Collier AC, Eron JJ, Mellors JW. 2017. Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 13:1–21. doi:10.1371/journal.ppat.1006285. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

