Necroptosis in development and diseases
- PMID: 29593066
- PMCID: PMC5900707
- DOI: 10.1101/gad.312561.118
Necroptosis in development and diseases
Abstract
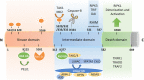
Necroptosis, a form of regulated necrotic cell death mediated by RIPK1 (receptor-interacting protein kinase 1) kinase activity, RIPK3, and MLKL (mixed-lineage kinase domain-like pseudokinase), can be activated under apoptosis-deficient conditions. Modulating the activation of RIPK1 by ubiquitination and phosphorylation is critical to control both necroptosis and apoptosis. Mutant mice with kinase-dead RIPK1 or RIPK3 and MLKL deficiency show no detrimental phenotype in regard to development and adult homeostasis. However, necroptosis and apoptosis can be activated in response to various mutations that result in the abortion of the defective embryos and human inflammatory and neurodegenerative pathologies. RIPK1 inhibition represents a key therapeutic strategy for treatment of diseases where blocking both necroptosis and apoptosis can be beneficial.
Keywords: MLKL; RIPK1; RIPK3; apoptosis; necroptosis.
© 2018 Shan et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Aggarwal BB. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3: 745–756. - PubMed
-
- Aigelsreiter A, Haybaeck J, Schauer S, Kiesslich T, Bettermann K, Griessbacher A, Stojakovic T, Bauernhofer T, Samonigg H, Kornprat P, et al. 2012. NEMO expression in human hepatocellular carcinoma and its association with clinical outcome. Hum Pathol 43: 1012–1019. - PubMed
-
- Alcamo E, Mizgerd JP, Horwitz BH, Bronson R, Beg AA, Scott M, Doerschuk CM, Hynes RO, Baltimore D. 2001. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-κB in leukocyte recruitment. J Immunol 167: 1592–1600. - PubMed
-
- Alvarez-Diaz S, Dillon CP, Lalaoui N, Tanzer MC, Rodriguez DA, Lin A, Lebois M, Hakem R, Josefsson EC, O'Reilly LA, et al. 2016. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45: 513–526. - PMC - PubMed
-
- Arsenescu R, Bruno ME, Rogier EW, Stefka AT, McMahan AE, Wright TB, Nasser MS, de Villiers WJ, Kaetzel CS. 2008. Signature biomarkers in Crohn's disease: toward a molecular classification. Mucosal Immunol 1: 399–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
