Vps74 Connects the Golgi Apparatus and Telomeres in Saccharomyces cerevisiae
- PMID: 29593073
- PMCID: PMC5940170
- DOI: 10.1534/g3.118.200172
Vps74 Connects the Golgi Apparatus and Telomeres in Saccharomyces cerevisiae
Abstract
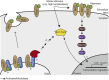
In mammalian cell culture, the Golgi apparatus fragment upon DNA damage. GOLPH3, a Golgi component, is a phosphorylation target of DNA-PK after DNA damage and contributes to Golgi fragmentation. The function of the yeast (Saccharomyces cerevisiae) ortholog of GOLPH3, Vps74, in the DNA damage response has been little studied, although genome-wide screens suggested a role at telomeres. In this study we investigated the role of Vps74 at telomeres and in the DNA damage response. We show that Vps74 decreases the fitness of telomere defective cdc13-1 cells and contributes to the fitness of yku70Δ cells. Importantly, loss of Vps74 in yku70Δ cells exacerbates the temperature dependent growth defects of these cells in a Chk1 and Mec1-dependent manner. Furthermore, Exo1 reduces the fitness of vps74Δ yku70Δ cells suggesting that ssDNA contributes to the fitness defects of vps74Δ yku70Δ cells. Systematic genetic interaction analysis of vps74Δ, yku70Δ and yku70Δ vps74Δ cells suggests that vps74Δ causes a milder but similar defect to that seen in yku70Δ cells. vps74Δ cells have slightly shorter telomeres and loss of VPS74 in yku70Δ or mre11Δ cells further shortens the telomeres of these cells. Interestingly, loss of Vps74 leads to increased levels of Stn1, a partner of Cdc13 in the CST telomere capping complex. Overexpression of Stn1 was previously shown to cause telomere shortening, suppression of cdc13-1 and enhancement of yku70Δ growth defects, suggesting that increased levels of Stn1 may be the route by which Vps74 affects telomere function. These results establish Vps74 as a novel regulator of telomere biology.
Keywords: Golgi; QFA; Saccharomyces cerevisiae; Stn1; Vps74; telomere.
Copyright © 2018 Rodrigues et al.
Figures


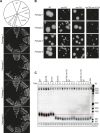
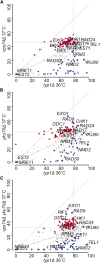
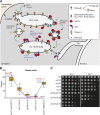
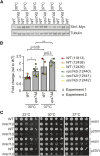
References
-
- Adams A., Gottschling D. E., Kaiser C. A., Stearns T., 1997. Methods in Yeast Genetics, Cold Spring Harbor Laboratory Press, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
