Thalidomide Reduces Hemorrhage of Brain Arteriovenous Malformations in a Mouse Model
- PMID: 29593101
- PMCID: PMC5916043
- DOI: 10.1161/STROKEAHA.117.020356
Thalidomide Reduces Hemorrhage of Brain Arteriovenous Malformations in a Mouse Model
Abstract
Background and purpose: Brain arteriovenous malformation (bAVM) is an important risk factor for intracranial hemorrhage. Current treatments for bAVM are all associated with considerable risks. There is no safe method to prevent bAVM hemorrhage. Thalidomide reduces nose bleeding in patients with hereditary hemorrhagic telangiectasia, an inherited disorder characterized by vascular malformations. In this study, we tested whether thalidomide and its less toxic analog, lenalidomide, reduce bAVM hemorrhage using a mouse model.
Methods: bAVMs were induced through induction of brain focal activin-like kinase 1 (Alk1, an AVM causative gene) gene deletion and angiogenesis in adult Alk1-floxed mice. Thalidomide was injected intraperitoneally twice per week for 6 weeks, starting either 2 or 8 weeks after AVM induction. Lenalidomide was injected intraperitoneally daily starting 8 weeks after AVM induction for 6 weeks. Brain samples were collected at the end of the treatments for morphology, mRNA, and protein analyses. The influence of Alk1 downregulation on PDGFB (platelet-derived growth factor B) expression was also studied on cultured human brain microvascular endothelial cells. The effect of PDGFB in mural cell recruitment in bAVM was explored by injection of a PDGFB overexpressing lentiviral vector to the mouse brain.
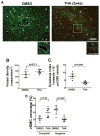
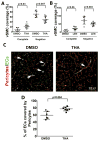
Results: Thalidomide or lenalidomide treatment reduced the number of dysplastic vessels and hemorrhage and increased mural cell (vascular smooth muscle cells and pericytes) coverage in the bAVM lesion. Thalidomide reduced the burden of CD68+ cells and the expression of inflammatory cytokines in the bAVM lesions. PDGFB expression was reduced in ALK1-knockdown human brain microvascular endothelial cells and in mouse bAVM lesion. Thalidomide increased Pdgfb expression in bAVM lesion. Overexpression of PDGFB mimicked the effect of thalidomide.
Conclusions: Thalidomide and lenalidomide improve mural cell coverage of bAVM vessels and reduce bAVM hemorrhage, which is likely through upregulation of Pdgfb expression.
Keywords: arteriovenous malformation; brain; endothelial cells; hemorrhage; thalidomide.
© 2018 American Heart Association, Inc.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




References
-
- Sanders RD, Avidan MS. Postoperative cognitive trajectories in adults: The role of inflammatory processes. Anesthesiology. 2013;118:484–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

