Evidence for functional pre-coupled complexes of receptor heteromers and adenylyl cyclase
- PMID: 29593213
- PMCID: PMC5871782
- DOI: 10.1038/s41467-018-03522-3
Evidence for functional pre-coupled complexes of receptor heteromers and adenylyl cyclase
Abstract
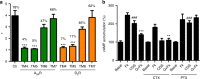
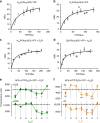
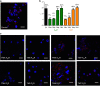
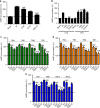
G protein-coupled receptors (GPCRs), G proteins and adenylyl cyclase (AC) comprise one of the most studied transmembrane cell signaling pathways. However, it is unknown whether the ligand-dependent interactions between these signaling molecules are based on random collisions or the rearrangement of pre-coupled elements in a macromolecular complex. Furthermore, it remains controversial whether a GPCR homodimer coupled to a single heterotrimeric G protein constitutes a common functional unit. Using a peptide-based approach, we here report evidence for the existence of functional pre-coupled complexes of heteromers of adenosine A2A receptor and dopamine D2 receptor homodimers coupled to their cognate Gs and Gi proteins and to subtype 5 AC. We also demonstrate that this macromolecular complex provides the necessary frame for the canonical Gs-Gi interactions at the AC level, sustaining the ability of a Gi-coupled GPCR to counteract AC activation mediated by a Gs-coupled GPCR.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Oligomerization of G protein-coupled receptors: Still doubted?Prog Mol Biol Transl Sci. 2020;169:297-321. doi: 10.1016/bs.pmbts.2019.11.006. Epub 2019 Dec 12. Prog Mol Biol Transl Sci. 2020. PMID: 31952690 Free PMC article. Review.
-
Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer.Neuropharmacology. 2016 May;104:154-60. doi: 10.1016/j.neuropharm.2015.05.028. Epub 2015 Jun 4. Neuropharmacology. 2016. PMID: 26051403 Free PMC article. Review.
-
Intracellular calcium levels determine differential modulation of allosteric interactions within G protein-coupled receptor heteromers.Chem Biol. 2014 Nov 20;21(11):1546-56. doi: 10.1016/j.chembiol.2014.10.004. Chem Biol. 2014. PMID: 25457181 Free PMC article.
-
Type II adenylylcyclase integrates coincident signals from Gs, Gi, and Gq.J Biol Chem. 1993 Jul 5;268(19):13900-5. J Biol Chem. 1993. PMID: 8390980
-
Multiple signaling routes involved in the regulation of adenylyl cyclase and extracellular regulated kinase by dopamine D(2) and D(3) receptors.Pharmacol Res. 2013 Jan;67(1):31-41. doi: 10.1016/j.phrs.2012.09.012. Epub 2012 Oct 8. Pharmacol Res. 2013. PMID: 23059541
Cited by
-
AKAP5 complex facilitates purinergic modulation of vascular L-type Ca2+ channel CaV1.2.Nat Commun. 2020 Oct 20;11(1):5303. doi: 10.1038/s41467-020-18947-y. Nat Commun. 2020. PMID: 33082339 Free PMC article.
-
Pharmacological targeting of G protein-coupled receptor heteromers.Pharmacol Res. 2022 Nov;185:106476. doi: 10.1016/j.phrs.2022.106476. Epub 2022 Sep 28. Pharmacol Res. 2022. PMID: 36182040 Free PMC article.
-
Mechanistic insight into the impact of a bivalent ligand on the structure and dynamics of a GPCR oligomer.Comput Struct Biotechnol J. 2022 Feb 4;20:925-936. doi: 10.1016/j.csbj.2022.01.016. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35242285 Free PMC article.
-
Follicle-stimulating hormone signaling in Sertoli cells: a licence to the early stages of spermatogenesis.Reprod Biol Endocrinol. 2022 Jul 2;20(1):97. doi: 10.1186/s12958-022-00971-w. Reprod Biol Endocrinol. 2022. PMID: 35780146 Free PMC article. Review.
-
Oligomerization of G protein-coupled receptors: Still doubted?Prog Mol Biol Transl Sci. 2020;169:297-321. doi: 10.1016/bs.pmbts.2019.11.006. Epub 2019 Dec 12. Prog Mol Biol Transl Sci. 2020. PMID: 31952690 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

