Lung Topology Characteristics in patients with Chronic Obstructive Pulmonary Disease
- PMID: 29593257
- PMCID: PMC5871819
- DOI: 10.1038/s41598-018-23424-0
Lung Topology Characteristics in patients with Chronic Obstructive Pulmonary Disease
Abstract
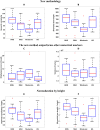
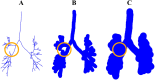
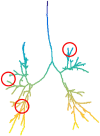
Quantitative features that can currently be obtained from medical imaging do not provide a complete picture of Chronic Obstructive Pulmonary Disease (COPD). In this paper, we introduce a novel analytical tool based on persistent homology that extracts quantitative features from chest CT scans to describe the geometric structure of the airways inside the lungs. We show that these new radiomic features stratify COPD patients in agreement with the GOLD guidelines for COPD and can distinguish between inspiratory and expiratory scans. These CT measurements are very different to those currently in use and we demonstrate that they convey significant medical information. The results of this study are a proof of concept that topological methods can enhance the standard methodology to create a finer classification of COPD and increase the possibilities of more personalized treatment.
Conflict of interest statement
The authors declare no competing interests.
Figures


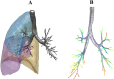

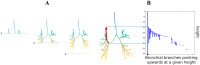
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

