Kaempferol-immobilized titanium dioxide promotes formation of new bone: effects of loading methods on bone marrow stromal cell differentiation in vivo and in vitro
- PMID: 29593412
- PMCID: PMC5865554
- DOI: 10.2147/IJN.S150786
Kaempferol-immobilized titanium dioxide promotes formation of new bone: effects of loading methods on bone marrow stromal cell differentiation in vivo and in vitro
Abstract
Background: Surface modification of titanium dioxide (TiO2) implants promotes bone formation and shortens the osseointegration period. Kaempferol is a flavonoid that has the capacity to promote osteogenic differentiation in bone marrow stromal cells. The aim of this study was to promote bone formation around kaempferol immobilized on TiO2 implants.
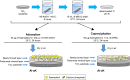
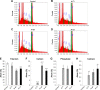
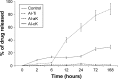
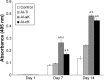
Methods: There were four experimental groups. Alkali-treated TiO2 samples (implants and discs) were used as a control and immersed in Dulbecco's phosphate-buffered saline (DPBS) (Al-Ti). For the coprecipitation sample (Al-cK), the control samples were immersed in DPBS containing 50 µg kaempferol/100% ethanol. For the adsorption sample (Al-aK), 50 µg kaempferol/100% ethanol was dropped onto control samples. The surface topography of the TiO2 implants was observed by scanning electron microscopy with energy-dispersive X-ray spectroscopy, and a release assay was performed. For in vitro experiments, rat bone marrow stromal cells (rBMSCs) were cultured on each of the TiO2 samples to analyze cell proliferation, alkaline phosphatase activity, calcium deposition, and osteogenic differentiation. For in vivo experiments, TiO2 implants placed on rat femur bones were analyzed for bone-implant contact by histological methods.
Results: Kaempferol was detected on the surface of Al-cK and Al-aK. The results of the in vitro study showed that rBMSCs cultured on Al-cK and Al-aK promoted alkaline phosphatase activity, calcium deposition, and osteogenic differentiation. The in vivo histological analysis revealed that Al-cK and Al-aK stimulated new bone formation around implants.
Conclusion: TiO2 implant-immobilized kaempferol may be an effective tool for bone regeneration around dental implants.
Keywords: biomaterial; kaempferol; surface treatment; titanium implant.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
A new application of cell-free bone regeneration: immobilizing stem cells from human exfoliated deciduous teeth-conditioned medium onto titanium implants using atmospheric pressure plasma treatment.Stem Cell Res Ther. 2015 Jun 19;6(1):124. doi: 10.1186/s13287-015-0114-1. Stem Cell Res Ther. 2015. PMID: 26088364 Free PMC article.
-
Enhanced Osseointegration of Titanium Implants by Surface Modification with Silicon-doped Titania Nanotubes.Int J Nanomedicine. 2020 Nov 3;15:8583-8594. doi: 10.2147/IJN.S270311. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33173295 Free PMC article.
-
Effects of a hybrid micro/nanorod topography-modified titanium implant on adhesion and osteogenic differentiation in rat bone marrow mesenchymal stem cells.Int J Nanomedicine. 2013;8:257-65. doi: 10.2147/IJN.S39357. Epub 2013 Jan 11. Int J Nanomedicine. 2013. PMID: 23345973 Free PMC article.
-
Nanostructured Titanium Implant Surface Facilitating Osseointegration from Protein Adsorption to Osteogenesis: The Example of TiO2 NTAs.Int J Nanomedicine. 2022 Apr 29;17:1865-1879. doi: 10.2147/IJN.S362720. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35518451 Free PMC article. Review.
-
Growth factor-functionalized titanium implants for enhanced bone regeneration: A review.Int J Biol Macromol. 2024 Aug;274(Pt 2):133153. doi: 10.1016/j.ijbiomac.2024.133153. Epub 2024 Jun 17. Int J Biol Macromol. 2024. PMID: 38897500 Review.
Cited by
-
Flavonoid-Loaded Biomaterials in Bone Defect Repair.Molecules. 2023 Sep 30;28(19):6888. doi: 10.3390/molecules28196888. Molecules. 2023. PMID: 37836731 Free PMC article. Review.
-
The Osteoprotective Effects Of Kaempferol: The Evidence From In Vivo And In Vitro Studies.Drug Des Devel Ther. 2019 Oct 7;13:3497-3514. doi: 10.2147/DDDT.S227738. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31631974 Free PMC article. Review.
-
The effect of kaempferol on the dentin bonding stability through matrix metalloproteinases inhibition and collagen crosslink in dentin biomodification.J Dent Sci. 2023 Jul;18(3):1023-1030. doi: 10.1016/j.jds.2022.12.002. Epub 2022 Dec 14. J Dent Sci. 2023. PMID: 37404650 Free PMC article.
-
Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling.BMC Cancer. 2021 Apr 12;21(1):396. doi: 10.1186/s12885-021-08158-z. BMC Cancer. 2021. PMID: 33845796 Free PMC article.
-
Effect and mechanism of propranolol on promoting osteogenic differentiation and early implant osseointegration.Int J Mol Med. 2021 Oct;48(4):191. doi: 10.3892/ijmm.2021.5024. Epub 2021 Aug 20. Int J Mol Med. 2021. PMID: 34414453 Free PMC article.
References
-
- Brånemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38(2):175–181. - PubMed
-
- Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50(3):399–410. - PubMed
-
- Vandamme K, Holy X, Bensidhoum M, et al. In vivo molecular evidence of delayed titanium implant osseointegration in compromised bone. Biomaterials. 2011;32(14):3547–3554. - PubMed
-
- Eom TG, Jeon GR, Jeong CM, et al. Experimental study of bone response to hydroxyapatite coating implants: bone-implant contact and removal torque test. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:411–418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

