The Interaction of Pre-programmed Eye Movements With the Vestibulo-Ocular Reflex
- PMID: 29593506
- PMCID: PMC5855878
- DOI: 10.3389/fnsys.2018.00004
The Interaction of Pre-programmed Eye Movements With the Vestibulo-Ocular Reflex
Abstract
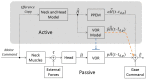
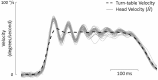
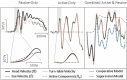
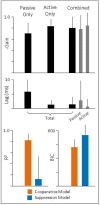
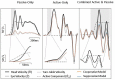
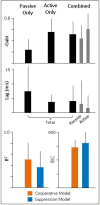
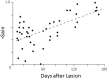
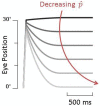
The Vestibulo-Ocular Reflex (VOR) works to stabilize gaze during unexpected head movements. However, even subjects who lack a VOR (e.g., vestibulopathic patients) can achieve gaze stability during planned head movements by using pre-programmed eye movements (PPEM). The extent to which PPEM are used by healthy intact subjects and how they interact with the VOR is still unclear. We propose a model of gaze stabilization which makes several claims: (1) the VOR provides ocular stability during unexpected (i.e., passive) head movements; (2) PPEM are used by both healthy and vestibulopathic subjects during planned (i.e., active) head movements; and (3) when a passive perturbation interrupts an active head movement in intact animals (i.e., combined passive and active head movement) the VOR works with PPEM to provide compensation. First, we show how our model can reconcile some seemingly conflicting findings in earlier literature. We then test the above-mentioned predictions against data we collected from both healthy and vestibular-lesioned guinea pigs. We found that (1) vestibular-lesioned animals showed a dramatic decrease in compensatory eye movements during passive head movements, (2) both populations showed improved ocular compensation during active vs. passive head movements, and (3) during combined active and passive head movements, eye movements compensated for both the active and passive component of head velocity. These results support our hypothesis that while the VOR provides compensation during passive head movements, PPEM are used by both intact and lesioned subjects during active movements and further, that PPEM work together with the VOR to achieve gaze stability.
Keywords: Vestibulo-Ocular Reflex; adaptation; biological; efference copy; gaze stabilization; internal model.
Figures

Similar articles
-
Gaze stabilization in chronic vestibular-loss and in cerebellar ataxia: interactions of feedforward and sensory feedback mechanisms.J Vestib Res. 2014;24(5-6):425-31. doi: 10.3233/VES-140538. J Vestib Res. 2014. PMID: 25564085 Clinical Trial.
-
Vestibuloocular reflex signal modulation during voluntary and passive head movements.J Neurophysiol. 2002 May;87(5):2337-57. doi: 10.1152/jn.2002.87.5.2337. J Neurophysiol. 2002. PMID: 11976372
-
Enhancement of the vestibulo-ocular reflex by prior eye movements.J Neurophysiol. 1999 Jun;81(6):2884-92. doi: 10.1152/jn.1999.81.6.2884. J Neurophysiol. 1999. PMID: 10368405 Clinical Trial.
-
Getting ahead of oneself: anticipation and the vestibulo-ocular reflex.Neuroscience. 2013 Apr 16;236:210-9. doi: 10.1016/j.neuroscience.2012.12.032. Epub 2013 Jan 29. Neuroscience. 2013. PMID: 23370320 Free PMC article. Review.
-
Anticipatory eye movements stabilize gaze during self-generated head movements.Ann N Y Acad Sci. 2011 Sep;1233:219-25. doi: 10.1111/j.1749-6632.2011.06165.x. Ann N Y Acad Sci. 2011. PMID: 21950997 Free PMC article. Review.
Cited by
-
Role of locomotor efference copy in vertebrate gaze stabilization.Front Neural Circuits. 2022 Dec 9;16:1040070. doi: 10.3389/fncir.2022.1040070. eCollection 2022. Front Neural Circuits. 2022. PMID: 36569798 Free PMC article. Review.
-
Retinal motion statistics during natural locomotion.Elife. 2023 May 3;12:e82410. doi: 10.7554/eLife.82410. Elife. 2023. PMID: 37133442 Free PMC article.
-
Active vision in freely moving marmosets using head-mounted eye tracking.bioRxiv [Preprint]. 2024 Nov 21:2024.05.11.593707. doi: 10.1101/2024.05.11.593707. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2412954122. doi: 10.1073/pnas.2412954122. PMID: 38766147 Free PMC article. Updated. Preprint.
-
Active vision in freely moving marmosets using head-mounted eye tracking.Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2412954122. doi: 10.1073/pnas.2412954122. Epub 2025 Feb 3. Proc Natl Acad Sci U S A. 2025. PMID: 39899712 Free PMC article.
-
Retinal optic flow during natural locomotion.PLoS Comput Biol. 2022 Feb 22;18(2):e1009575. doi: 10.1371/journal.pcbi.1009575. eCollection 2022 Feb. PLoS Comput Biol. 2022. PMID: 35192614 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

