The Aging of Iron Man
- PMID: 29593525
- PMCID: PMC5857593
- DOI: 10.3389/fnagi.2018.00065
The Aging of Iron Man
Abstract
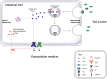
Brain iron is tightly regulated by a multitude of proteins to ensure homeostasis. Iron dyshomeostasis has become a molecular signature associated with aging which is accompanied by progressive decline in cognitive processes. A common theme in neurodegenerative diseases where age is the major risk factor, iron dyshomeostasis coincides with neuroinflammation, abnormal protein aggregation, neurodegeneration, and neurobehavioral deficits. There is a great need to determine the mechanisms governing perturbations in iron metabolism, in particular to distinguish between physiological and pathological aging to generate fruitful therapeutic targets for neurodegenerative diseases. The aim of the present review is to focus on the age-related alterations in brain iron metabolism from a cellular and molecular biology perspective, alongside genetics, and neuroimaging aspects in man and rodent models, with respect to normal aging and neurodegeneration. In particular, the relationship between iron dyshomeostasis and neuroinflammation will be evaluated, as well as the effects of systemic iron overload on the brain. Based on the evidence discussed here, we suggest a synergistic use of iron-chelators and anti-inflammatories as putative anti-brain aging therapies to counteract pathological aging in neurodegenerative diseases.
Keywords: Alzheimer's disease; Parkinson's disease; aging; brain iron; iron overload; iron regulation; neurodegenerative diseases; neuroinflammation.
Figures



References
-
- Aldred A. R., Grimes A., Schreiber G., Mercer J. F. (1987). Rat ceruloplasmin. molecular cloning and gene expression in liver, choroid plexus, yolk sac, placenta, and testis. J. Biol. Chem. 262, 2875–2878. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

