Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design
- PMID: 29593527
- PMCID: PMC5854945
- DOI: 10.3389/fphar.2018.00128
Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design
Abstract
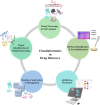
The primary goal of rational drug discovery is the identification of selective ligands which act on single or multiple drug targets to achieve the desired clinical outcome through the exploration of total chemical space. To identify such desired compounds, computational approaches are necessary in predicting their drug-like properties. G Protein-Coupled Receptors (GPCRs) represent one of the largest and most important integral membrane protein families. These receptors serve as increasingly attractive drug targets due to their relevance in the treatment of various diseases, such as inflammatory disorders, metabolic imbalances, cardiac disorders, cancer, monogenic disorders, etc. In the last decade, multitudes of three-dimensional (3D) structures were solved for diverse GPCRs, thus referring to this period as the "golden age for GPCR structural biology." Moreover, accumulation of data about the chemical properties of GPCR ligands has garnered much interest toward the exploration of GPCR chemical space. Due to the steady increase in the structural, ligand, and functional data of GPCRs, several cheminformatics approaches have been implemented in its drug discovery pipeline. In this review, we mainly focus on the cheminformatics-based paradigms in GPCR drug discovery. We provide a comprehensive view on the ligand- and structure-based cheminformatics approaches which are best illustrated via GPCR case studies. Furthermore, an appropriate combination of ligand-based knowledge with structure-based ones, i.e., integrated approach, which is emerging as a promising strategy for cheminformatics-based GPCR drug design is also discussed.
Keywords: GPCR; cheminformatics; drug discovery; ligand-based drug design; structure-based drug design.
Figures



References
-
- Bajorath J. (2004). Chemoinformatics: Concepts, Methods, and Tools for Drug Discovery. New Jersey, NJ: Humana Press.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

