Mechanistic Role of Reactive Oxygen Species and Therapeutic Potential of Antioxidants in Denervation- or Fasting-Induced Skeletal Muscle Atrophy
- PMID: 29593571
- PMCID: PMC5861206
- DOI: 10.3389/fphys.2018.00215
Mechanistic Role of Reactive Oxygen Species and Therapeutic Potential of Antioxidants in Denervation- or Fasting-Induced Skeletal Muscle Atrophy
Abstract
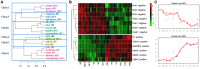
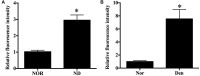
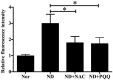
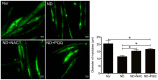
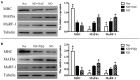
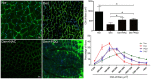
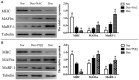
Skeletal muscle atrophy occurs under various conditions, such as disuse, denervation, fasting, aging, and various diseases. Although the underlying molecular mechanisms are still not fully understood, skeletal muscle atrophy is closely associated with reactive oxygen species (ROS) overproduction. In this study, we aimed to investigate the involvement of ROS in skeletal muscle atrophy from the perspective of gene regulation, and further examine therapeutic effects of antioxidants on skeletal muscle atrophy. Microarray data showed that the gene expression of many positive regulators for ROS production were up-regulated and the gene expression of many negative regulators for ROS production were down-regulated in mouse soleus muscle atrophied by denervation (sciatic nerve injury). The ROS level was significantly increased in denervated mouse soleus muscle or fasted C2C12 myotubes that had suffered from fasting (nutrient deprivation). These two muscle samples were then treated with N-acetyl-L-cysteine (NAC, a clinically used antioxidant) or pyrroloquinoline quinone (PQQ, a naturally occurring antioxidant), respectively. As compared to non-treatment, both NAC and PQQ treatment (1) reversed the increase in the ROS level in two muscle samples; (2) attenuated the reduction in the cross-sectional area (CSA) of denervated mouse muscle or in the diameter of fasted C2C12 myotube; (3) increased the myosin heavy chain (MHC) level and decreased the muscle atrophy F-box (MAFbx) and muscle-specific RING finger-1 (MuRF-1) levels in two muscle samples. Collectively, these results suggested that an increased ROS level was, at least partly, responsible for denervation- or fasting-induced skeletal muscle atrophy, and antioxidants might resist the atrophic effect via ROS-related mechanisms.
Keywords: N-acetyl-L-cysteine; antioxidant therapy; microarray; pyrroloquinoline quinone; reactive oxidative species; skeletal muscle atrophy.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

