The Use of Biophysical Flow Models in the Surgical Management of Patients Affected by Chronic Thromboembolic Pulmonary Hypertension
- PMID: 29593574
- PMCID: PMC5859070
- DOI: 10.3389/fphys.2018.00223
The Use of Biophysical Flow Models in the Surgical Management of Patients Affected by Chronic Thromboembolic Pulmonary Hypertension
Abstract
Introduction: Chronic Thromboembolic Pulmonary Hypertension (CTEPH) results from progressive thrombotic occlusion of the pulmonary arteries. It is treated by surgical removal of the occlusion, with success rates depending on the degree of microvascular remodeling. Surgical eligibility is influenced by the contributions of both the thrombus occlusion and microvasculature remodeling to the overall vascular resistance. Assessing this is challenging due to the high inter-individual variability in arterial morphology and physiology. We investigated the potential of patient-specific computational flow modeling to quantify pressure gradients in the pulmonary arteries of CTEPH patients to assist the decision-making process for surgical eligibility. Methods: Detailed segmentations of the pulmonary arteries were created from postoperative chest Computed Tomography scans of three CTEPH patients. A focal stenosis was included in the original geometry to compare the pre- and post-surgical hemodynamics. Three-dimensional flow simulations were performed on each morphology to quantify velocity-dependent pressure changes using a finite element solver coupled to terminal 2-element Windkessel models. In addition to transient flow simulations, a parametric modeling approach based on constant flow simulations is also proposed as faster technique to estimate relative pressure drops through the proximal pulmonary vasculature. Results: An asymmetrical flow split between left and right pulmonary arteries was observed in the stenosed models. Removing the proximal obstruction resulted in a reduction of the right-left pressure imbalance of up to 18%. Changes were also observed in the wall shear stresses and flow topology, where vortices developed in the stenosed model while the non-stenosed retained a helical flow. The predicted pressure gradients from constant flow simulations were consistent with the ones measured in the transient flow simulations. Conclusion: This study provides a proof of concept that patient-specific computational modeling can be used as a noninvasive tool for assisting surgical decisions in CTEPH based on hemodynamics metrics. Our technique enables determination of the proximal relative pressure, which could subsequently be compared to the total pressure drop to determine the degree of distal and proximal vascular resistance. In the longer term this approach has the potential to form the basis for a more quantitative classification system of CTEPH types.
Keywords: CTEPH; HPC-based computational modeling; biophysical flow modeling; computational physiology; patient specific computational modeling.
Figures
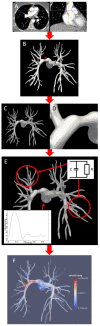
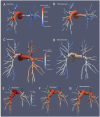


References
-
- Arthurs C. J., Agarwal P., John A. V., Dorfman A. L., Grifka R. G., Figueroa C. A. (2017). Reproducing patient-specific hemodynamics in the Blalock-Taussig circulation using a flexible multi-domain simulation framework: applications for optimal shunt design. Front. Pediatr. 5:78. 10.3389/fped.2017.00078 - DOI - PMC - PubMed
-
- Arthurs C. J., Lau K. D., Asrress K. N., Redwood S. R., Figueroa C. A. (2016). A mathematical model of coronary blood flow control: simulation of patient-specific three-dimensional hemodynamics during exercise. Am. J. Physiol. Heart Circ. Physiol. 310, H1242–H1258. 10.1152/ajpheart.00517.2015 - DOI - PMC - PubMed
-
- Azarian R., Wartski M., Collignon M. A., Parent F., Hervé P., Sors H., et al. . (1997). Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. J. Nucl. Med. 38, 980–993. - PubMed
-
- Boussel L., Rayz V., McCulloch C., Martin A., Acevedo-Bolton G., Lawton M., et al. . (2008). Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 39, 2997–3002. 10.1161/STROKEAHA.108.521617 - DOI - PMC - PubMed
-
- Buelow T., Wiemker R., Blaffert T., Lorenz C., Renisch S. (2005). Automatic extraction of the pulmonary artery tree from multi-slice CT data. Proc. SPIE. 5746, 730–740. 10.1117/12.595286 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

