Pattern of Use of Biosimilar and Originator Somatropin in Italy: A Population-Based Multiple Databases Study During the Years 2009-2014
- PMID: 29593655
- PMCID: PMC5859012
- DOI: 10.3389/fendo.2018.00095
Pattern of Use of Biosimilar and Originator Somatropin in Italy: A Population-Based Multiple Databases Study During the Years 2009-2014
Abstract
Purpose: Somatropin [recombinant growth hormone (rGH)] is approved in children and adults for several conditions involving growth disturbances and the corresponding biosimilar is available in Italy since 2006. No population-based data are available on the pattern of rGH use in Italian clinical practice. This study aimed at exploring the pattern of biosimilar and originator rGH use in six Italian centers, where different policy interventions promoted biosimilar use.
Methods: This population-based, drug-utilization study was conducted in the years 2009-2014, using administrative databases of Umbria, Tuscany, and Lazio Regions and Local Health Units of Caserta, Treviso, and Palermo. Naïve rGH users were characterized, and prevalence of use and discontinuation were assessed over time.
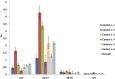
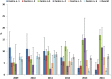
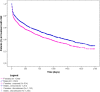
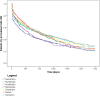
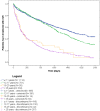
Results: Among 6,785 patients treated with rGH during the study years, 4,493 (66.2%) were naïve users (males/females = 1.3), mostly affected by GH deficiency. The prevalence of rGH use increased from 2009 to 2010, remaining stable thereafter, but it was heterogeneous across centers (twofold higher prevalence of use in center n.2 than centers n.4 and 1 in 2014). Biosimilar rGH uptake increased over time but was low (7.8% in 2014) and heterogeneous as well. Discontinuation of rGH therapy occurred in 54.0% of naïve users, more frequently in females than males (58.1 vs. 50.9%). During the first year of treatment, discontinuation was frequent (39.9%), but no statistically significant differences were observed in treatment persistence for biosimilar vs. originator rGH (p > 0.05).
Conclusion: Geographical heterogeneity in the prevalence of rGH use was observed. Similarly, the biosimilar rGH uptake was low and variable across centers. Post-marketing monitoring is required to continuously monitor the benefit-risk profile of rGH, thus guaranteeing greater savings than only promoting lowest cost rGH.
Keywords: biosimilar; drug-utilization study; health-care administrative databases; pattern of use; somatropin.
Figures



Similar articles
-
How did the Introduction of Biosimilar Filgrastim Influence the Prescribing Pattern of Granulocyte Colony-Stimulating Factors? Results from a Multicentre, Population-Based Study, from Five Italian Centres in the Years 2009-2014.BioDrugs. 2016 Aug;30(4):295-306. doi: 10.1007/s40259-016-0175-4. BioDrugs. 2016. PMID: 27138636
-
How Much Are Biosimilars Used in Clinical Practice? A Retrospective Italian Population-Based Study of Erythropoiesis-Stimulating Agents in the Years 2009-2013.BioDrugs. 2015 Aug;29(4):275-84. doi: 10.1007/s40259-015-0132-7. BioDrugs. 2015. PMID: 26169209 Free PMC article.
-
Safety and effectiveness of biosimilar of Rituximab CT-P10 in the treatment of cryoglobulinemic vasculitis: the MARBLe study (Mixed cryoglobulinemiA Rituximab BiosimiLar).Intern Emerg Med. 2021 Jan;16(1):149-156. doi: 10.1007/s11739-020-02386-0. Epub 2020 Jun 10. Intern Emerg Med. 2021. PMID: 32524338
-
Patients Retransitioning from Biosimilar TNFα Inhibitor to the Corresponding Originator After Initial Transitioning to the Biosimilar: A Systematic Review.BioDrugs. 2022 Jan;36(1):27-39. doi: 10.1007/s40259-021-00508-4. Epub 2021 Dec 6. BioDrugs. 2022. PMID: 34870802 Free PMC article.
-
Drug Discontinuation in Studies Including a Switch From an Originator to a Biosimilar Monoclonal Antibody: A Systematic Literature Review.Clin Ther. 2019 Jan;41(1):155-173.e13. doi: 10.1016/j.clinthera.2018.11.002. Epub 2018 Dec 11. Clin Ther. 2019. PMID: 30551802
Cited by
-
How Have Intravitreal Anti-VEGF and Dexamethasone Implant Been Used in Italy? A Multiregional, Population-Based Study in the Years 2010-2016.Biomed Res Int. 2020 Jan 11;2020:7582763. doi: 10.1155/2020/7582763. eCollection 2020. Biomed Res Int. 2020. PMID: 31998798 Free PMC article.
-
Characteristics and Absolute Survival of Metastatic Colorectal Cancer Patients Treated With Biologics: A Real-World Data Analysis From Three European Countries.Front Oncol. 2021 Mar 5;11:630456. doi: 10.3389/fonc.2021.630456. eCollection 2021. Front Oncol. 2021. PMID: 33747950 Free PMC article.
-
Different Policy Measures and Practices between Swedish Counties Influence Market Dynamics: Part 2-Biosimilar and Originator Etanercept in the Outpatient Setting.BioDrugs. 2019 Jun;33(3):299-306. doi: 10.1007/s40259-019-00346-5. BioDrugs. 2019. PMID: 30945206 Free PMC article.
-
Healthcare Database Networks for Drug Regulatory Policies: International Workshop on the Canadian, US and Spanish Experience and Future Steps for Italy.Drug Saf. 2020 Jan;43(1):1-5. doi: 10.1007/s40264-019-00871-w. Drug Saf. 2020. PMID: 31641964 No abstract available.
-
Patient Preferences for Biologic and Biosimilar Osteoporosis Treatments in Colombia.Patient Prefer Adherence. 2020 Jun 23;14:1049-1064. doi: 10.2147/PPA.S250745. eCollection 2020. Patient Prefer Adherence. 2020. PMID: 32612354 Free PMC article.
References
-
- European Medicines Agency. Guideline on Similar Biological Medicinal Products. (2014). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- Italian Medicines Agency. National Report on Medicines Use in Italy. (2015). Available from: http://www.aifa.gov.it/sites/default/files/OsMed_2015_Eng.pdf
-
- Migliaretti G, Aimaretti G, Borraccino A, Bellone J, Vannelli S, Angeli A, et al. Incidence and prevalence rate estimation of GH treatment exposure in Piedmont pediatric population in the years 2002-2004: data from the GH Registry. J Endocrinol Invest (2006) 29:438–42.10.1007/BF03344127 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

