Assessment of Fecal Indicator Bacteria and Potential Pathogen Co-Occurrence at a Shellfish Growing Area
- PMID: 29593669
- PMCID: PMC5861211
- DOI: 10.3389/fmicb.2018.00384
Assessment of Fecal Indicator Bacteria and Potential Pathogen Co-Occurrence at a Shellfish Growing Area
Abstract
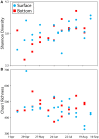
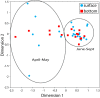
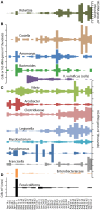
Routine monitoring of shellfish growing waters for bacteria indicative of human sewage pollution reveals little about the bacterial communities that co-occur with these indicators. This study investigated the bacterial community, potential pathogens, and fecal indicator bacteria in 40 water samples from a shellfish growing area in the Chesapeake Bay, USA. Bacterial community composition was quantified with deep sequencing of 16S rRNA gene amplicons, and absolute gene abundances were estimated with an internal standard (Thermus thermophilus genomes). Fecal coliforms were quantified by culture, and Vibrio vulnificus and V. parahaemolyticus with quantitative PCR. Fecal coliforms and V. vulnificus were detected in most samples, and a diverse assemblage of potential human pathogens were detected in all samples. These taxa followed two general patterns of abundance. Fecal coliforms and 16S rRNA genes for Enterobacteriaceae, Aeromonas, Arcobacter, Staphylococcus, and Bacteroides increased in abundance after a 1.3-inch rain event in May, and, for some taxa, after smaller rain events later in the season, suggesting that these are allochthonous organisms washed in from land. Clostridiaceae and Mycobacterium 16S rRNA gene abundances increased with day of the year and were not positively related to rainfall, suggesting that these are autochthonous organisms. Other groups followed both patterns, such as Legionella. Fecal coliform abundance did not correlate with most other taxa, but were extremely high following the large rainstorm in May when they co-occurred with a broad range of potential pathogen groups. V. vulnificus were absent during the large rainstorm, and did not correlate with 16S rRNA abundances of Vibrio spp. or most other taxa. These results highlight the complex nature of bacterial communities and the limited utility of using specific bacterial groups as indicators of pathogen presence.
Keywords: estuary; human health; microbiome; pathogens; shellfish closures.
Figures



Similar articles
-
Precipitation thresholds for fecal bacterial indicators in the Chesapeake Bay.Water Res. 2018 Aug 1;139:252-262. doi: 10.1016/j.watres.2018.04.004. Epub 2018 Apr 4. Water Res. 2018. PMID: 29655096
-
Microbial quality of wild shellfish in a tropical estuary subject to treated effluent discharge.Environ Res. 2020 Feb;181:108921. doi: 10.1016/j.envres.2019.108921. Epub 2019 Nov 14. Environ Res. 2020. PMID: 31757407
-
Climate relationships to fecal bacterial densities in Maryland shellfish harvest waters.Water Res. 2016 Feb 1;89:270-81. doi: 10.1016/j.watres.2015.11.055. Epub 2015 Nov 26. Water Res. 2016. PMID: 26689664
-
Relationships between Bacteroides 16S rRNA genetic markers and presence of bacterial enteric pathogens and conventional fecal indicators.Water Res. 2007 Aug;41(16):3615-28. doi: 10.1016/j.watres.2007.03.028. Epub 2007 May 15. Water Res. 2007. PMID: 17507075
-
Vibrio parahaemolyticus and Vibrio vulnificus in South America: water, seafood and human infections.J Appl Microbiol. 2016 Nov;121(5):1201-1222. doi: 10.1111/jam.13246. Epub 2016 Sep 8. J Appl Microbiol. 2016. PMID: 27459915 Review.
Cited by
-
Core and conditionally rare taxa as indicators of agricultural drainage ditch and stream health and function.BMC Microbiol. 2023 Mar 7;23(1):62. doi: 10.1186/s12866-023-02755-7. BMC Microbiol. 2023. PMID: 36882680 Free PMC article.
-
Pathogenic bacteria significantly increased under oxygen depletion in coastal waters: A continuous observation in the central Bohai Sea.Front Microbiol. 2022 Nov 21;13:1035904. doi: 10.3389/fmicb.2022.1035904. eCollection 2022. Front Microbiol. 2022. PMID: 36478871 Free PMC article.
-
Occurrence of Bacterial Pathogens and Human Noroviruses in Shellfish-Harvesting Areas and Their Catchments in France.Front Microbiol. 2018 Oct 11;9:2443. doi: 10.3389/fmicb.2018.02443. eCollection 2018. Front Microbiol. 2018. PMID: 30364306 Free PMC article.
-
Human Fecal Contamination Corresponds to Changes in the Freshwater Bacterial Communities of a Large River Basin.Microbiol Spectr. 2021 Oct 31;9(2):e0120021. doi: 10.1128/Spectrum.01200-21. Epub 2021 Sep 8. Microbiol Spectr. 2021. PMID: 34494860 Free PMC article.
-
The Prevalence of Arcobacteraceae in Aquatic Environments: A Systematic Review and Meta-Analysis.Pathogens. 2022 Feb 13;11(2):244. doi: 10.3390/pathogens11020244. Pathogens. 2022. PMID: 35215187 Free PMC article. Review.
References
-
- Ackerman D., Weisberg S. B. (2003). Relationship between rainfall and beach bacterial concentrations on Santa Monica Bay beaches. J. Water Health 1, 85–89. - PubMed
-
- APHA (1998). Standard Methods for the Examination of Water and Wastewater, 20th Edn. Washington, DC: American Public Health Association.
-
- Ashbolt N. J., Grabow W. O. K., Snozzi M. (2001). Indicators of microbial water quality, in Water Quality: Guidelines, Standards and Health, eds. Fewtrell L., Bartram J. (London: IWA Publishing; ), 289–315.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

