Signals Involved in Regulation of Hepatitis C Virus RNA Genome Translation and Replication
- PMID: 29593672
- PMCID: PMC5857606
- DOI: 10.3389/fmicb.2018.00395
Signals Involved in Regulation of Hepatitis C Virus RNA Genome Translation and Replication
Abstract
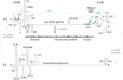
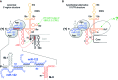
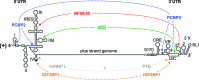
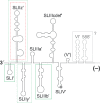
Hepatitis C virus (HCV) preferentially replicates in the human liver and frequently causes chronic infection, often leading to cirrhosis and liver cancer. HCV is an enveloped virus classified in the genus Hepacivirus in the family Flaviviridae and has a single-stranded RNA genome of positive orientation. The HCV RNA genome is translated and replicated in the cytoplasm. Translation is controlled by the Internal Ribosome Entry Site (IRES) in the 5' untranslated region (5' UTR), while also downstream elements like the cis-replication element (CRE) in the coding region and the 3' UTR are involved in translation regulation. The cis-elements controlling replication of the viral RNA genome are located mainly in the 5'- and 3'-UTRs at the genome ends but also in the protein coding region, and in part these signals overlap with the signals controlling RNA translation. Many long-range RNA-RNA interactions (LRIs) are predicted between different regions of the HCV RNA genome, and several such LRIs are actually involved in HCV translation and replication regulation. A number of RNA cis-elements recruit cellular RNA-binding proteins that are involved in the regulation of HCV translation and replication. In addition, the liver-specific microRNA-122 (miR-122) binds to two target sites at the 5' end of the viral RNA genome as well as to at least three additional target sites in the coding region and the 3' UTR. It is involved in the regulation of HCV RNA stability, translation and replication, thereby largely contributing to the hepatotropism of HCV. However, we are still far from completely understanding all interactions that regulate HCV RNA genome translation, stability, replication and encapsidation. In particular, many conclusions on the function of cis-elements in HCV replication have been obtained using full-length HCV genomes or near-full-length replicon systems. These include both genome ends, making it difficult to decide if a cis-element in question acts on HCV replication when physically present in the plus strand genome or in the minus strand antigenome. Therefore, it may be required to use reduced systems that selectively focus on the analysis of HCV minus strand initiation and/or plus strand initiation.
Keywords: HCV; cis-element; microRNA-122; replication; untranslated region.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

