Intestinal Microbiota Influences Non-intestinal Related Autoimmune Diseases
- PMID: 29593681
- PMCID: PMC5857604
- DOI: 10.3389/fmicb.2018.00432
Intestinal Microbiota Influences Non-intestinal Related Autoimmune Diseases
Abstract
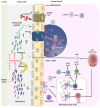
The human body is colonized by millions of microorganisms named microbiota that interact with our tissues in a cooperative and non-pathogenic manner. These microorganisms are present in the skin, gut, nasal, oral cavities, and genital tract. In fact, it has been described that the microbiota contributes to balancing the immune system to maintain host homeostasis. The gut is a vital organ where microbiota can influence and determine the function of cells of the immune system and contributes to preserve the wellbeing of the individual. Several articles have emphasized the connection between intestinal autoimmune diseases, such as Crohn's disease with dysbiosis or an imbalance in the microbiota composition in the gut. However, little is known about the role of the microbiota in autoimmune pathologies affecting other tissues than the intestine. This article focuses on what is known about the role that gut microbiota can play in the pathogenesis of non-intestinal autoimmune diseases, such as Grave's diseases, multiple sclerosis, type-1 diabetes, systemic lupus erythematosus, psoriasis, schizophrenia, and autism spectrum disorders. Furthermore, we discuss as to how metabolites derived from bacteria could be used as potential therapies for non-intestinal autoimmune diseases.
Keywords: CNS; autoimmune disease; gut; microbiome; microbiota; skin.
Figures

References
-
- Almonacid D. E., Kraal L., Ossandon F. J., Budovskaya Y. V., Cardenas J. P., Bik E. M., et al. . (2017). 16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome. PLoS ONE 12:e0176555. 10.1371/journal.pone.0176555 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

