Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis
- PMID: 29593709
- PMCID: PMC5859219
- DOI: 10.3389/fimmu.2018.00359
Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis
Abstract
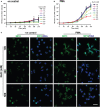
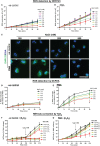
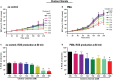
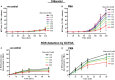
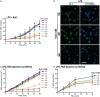
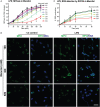
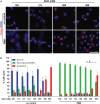
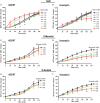
Tonicity of saline (NaCl) is important in regulating cellular functions and homeostasis. Hypertonic saline is administered to treat many inflammatory diseases, including cystic fibrosis. Excess neutrophil extracellular trap (NET) formation, or NETosis, is associated with many pathological conditions including chronic inflammation. Despite the known therapeutic benefits of hypertonic saline, its underlying mechanisms are not clearly understood. Therefore, we aimed to elucidate the effects of hypertonic saline in modulating NETosis. For this purpose, we purified human neutrophils and induced NETosis using agonists such as diacylglycerol mimetic phorbol myristate acetate (PMA), Gram-negative bacterial cell wall component lipopolysaccharide (LPS), calcium ionophores (A23187 and ionomycin from Streptomyces conglobatus), and bacteria (Pseudomonas aeruginosa and Staphylococcus aureus). We then analyzed neutrophils and NETs using Sytox green assay, immunostaining of NET components and apoptosis markers, confocal microscopy, and pH sensing reagents. This study found that hypertonic NaCl suppresses nicotinamide adenine dinucleotide phosphate oxidase (NADPH2 or NOX2)-dependent NETosis induced by agonists PMA, Escherichia coli LPS (0111:B4 and O128:B12), and P. aeruginosa. Hypertonic saline also suppresses LPS- and PMA- induced reactive oxygen species production. It was determined that supplementing H2O2 reverses the suppressive effect of hypertonic saline on NOX2-dependent NETosis. Many of the aforementioned suppressive effects were observed in the presence of equimolar concentrations of choline chloride and osmolytes (d-mannitol and d-sorbitol). This suggests that the mechanism by which hypertonic saline suppresses NOX2-dependent NETosis is via neutrophil dehydration. Hypertonic NaCl does not significantly alter the intracellular pH of neutrophils. We found that hypertonic NaCl induces apoptosis while suppressing NOX2-dependent NETosis. In contrast, hypertonic solutions do not suppress NOX2-independent NETosis. Although hypertonic saline partially suppresses ionomycin-induced NETosis, it enhances A23187-induced NETosis, and it does not alter S. aureus-induced NETosis. Overall, this study determined that hypertonic saline suppresses NOX2-dependent NETosis induced by several agonists; in contrast, it has variable effects on neutrophil death induced by NOX2-independent NETosis agonists. These findings are important in understanding the regulation of NETosis and apoptosis in neutrophils.
Keywords: Gram-negative and -positive bacteria-induced NETosis; NOX2-dependent NETosis; NaCl; cystic fibrosis; hypertonic saline; lipopolysaccharide-induced NETosis; neutrophil extracellular traps.
Figures
Similar articles
-
JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis.Sci Rep. 2017 Jun 13;7(1):3409. doi: 10.1038/s41598-017-03257-z. Sci Rep. 2017. PMID: 28611461 Free PMC article.
-
Histone Acetylation Promotes Neutrophil Extracellular Trap Formation.Biomolecules. 2019 Jan 18;9(1):32. doi: 10.3390/biom9010032. Biomolecules. 2019. PMID: 30669408 Free PMC article.
-
Histone Deacetylase Inhibitors Dose-Dependently Switch Neutrophil Death from NETosis to Apoptosis.Biomolecules. 2019 May 11;9(5):184. doi: 10.3390/biom9050184. Biomolecules. 2019. PMID: 31083537 Free PMC article.
-
Post-Translational Modifications in NETosis and NETs-Mediated Diseases.Biomolecules. 2019 Aug 14;9(8):369. doi: 10.3390/biom9080369. Biomolecules. 2019. PMID: 31416265 Free PMC article. Review.
-
How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation.Biomolecules. 2024 Oct 16;14(10):1307. doi: 10.3390/biom14101307. Biomolecules. 2024. PMID: 39456240 Free PMC article. Review.
Cited by
-
Blockade of caspase cascade overcomes malaria-associated acute respiratory distress syndrome in mice.Cell Death Dis. 2022 Feb 10;13(2):144. doi: 10.1038/s41419-022-04582-6. Cell Death Dis. 2022. PMID: 35145061 Free PMC article.
-
A novel cell-based assay for dynamically detecting neutrophil extracellular traps-induced lung epithelial injuries.Exp Cell Res. 2020 Sep 15;394(2):112101. doi: 10.1016/j.yexcr.2020.112101. Epub 2020 May 29. Exp Cell Res. 2020. PMID: 32474064 Free PMC article.
-
Neutrophil extracellular traps in intracerebral hemorrhage: implications for pathogenesis and therapeutic targets.Metab Brain Dis. 2023 Dec;38(8):2505-2520. doi: 10.1007/s11011-023-01268-6. Epub 2023 Jul 24. Metab Brain Dis. 2023. PMID: 37486436 Review.
-
Neutrophil extracellular trap formation index predicts occurrences of deep surgical site infection after laparotomy.Ann Transl Med. 2021 Sep;9(17):1373. doi: 10.21037/atm-21-1078. Ann Transl Med. 2021. PMID: 34733925 Free PMC article.
-
Impact of high- and low-flow nebulised saline on airway hydration and mucociliary transport.ERJ Open Res. 2023 Jun 12;9(3):00724-2022. doi: 10.1183/23120541.00724-2022. eCollection 2023 May. ERJ Open Res. 2023. PMID: 37313398 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

