Regenerating Immunotolerance in Multiple Sclerosis with Autologous Hematopoietic Stem Cell Transplant
- PMID: 29593711
- PMCID: PMC5857574
- DOI: 10.3389/fimmu.2018.00410
Regenerating Immunotolerance in Multiple Sclerosis with Autologous Hematopoietic Stem Cell Transplant
Abstract
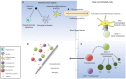
Multiple sclerosis (MS) is an inflammatory disorder of the central nervous system where evidence implicates an aberrant adaptive immune response in the accrual of neurological disability. The inflammatory phase of the disease responds to immunomodulation to varying degrees of efficacy; however, no therapy has been proven to arrest progression of disability. Recently, more intensive therapies, including immunoablation with autologous hematopoietic stem cell transplantation (AHSCT), have been offered as a treatment option to retard inflammatory disease, prior to patients becoming irreversibly disabled. Empirical clinical observations support the notion that the immune reconstitution (IR) that occurs following AHSCT is associated with a sustained therapeutic benefit; however, neither the pathogenesis of MS nor the mechanism by which AHSCT results in a therapeutic benefit has been clearly delineated. Although the antigenic target of the aberrant immune response in MS is not defined, accumulated data suggest that IR following AHSCT results in an immunotolerant state through deletion of pathogenic clones by a combination of direct ablation and induction of a lymphopenic state driving replicative senescence and clonal attrition. Restoration of immunoregulation is evidenced by changes in regulatory T cell populations following AHSCT and normalization of genetic signatures of immune homeostasis. Furthermore, some evidence exists that AHSCT may induce a rebooting of thymic function and regeneration of a diversified naïve T cell repertoire equipped to appropriately modulate the immune system in response to future antigenic challenge. In this review, we discuss the immunological mechanisms of IR therapies, focusing on AHSCT, as a means of recalibrating the dysfunctional immune response observed in MS.
Keywords: T cell receptor repertoire; alemtuzumab; autologous hematopoietic stem cell transplantation; cladribine; immune tolerance; lymphopenia-induced proliferation; multiple sclerosis.
Figures


References
-
- Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology (1993) 43(4):662–7.10.1212/WNL.43.4.662 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

