Effect of VH-VL Families in Pertuzumab and Trastuzumab Recombinant Production, Her2 and FcγIIA Binding
- PMID: 29593727
- PMCID: PMC5857972
- DOI: 10.3389/fimmu.2018.00469
Effect of VH-VL Families in Pertuzumab and Trastuzumab Recombinant Production, Her2 and FcγIIA Binding
Abstract
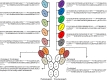
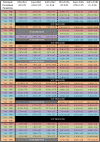
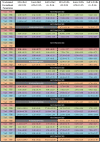
Many therapeutic antibodies are humanized from animal sources. In the humanization process, complementarity determining region grafting is tedious and highly prone to failure. With seven known VH families, and up to six known κ VL families, there are choices aplenty. However, the functions of these families remain largely enigmatic. To study the role of these V-region families, we made 84 recombinant combinations of the various VH and VL family whole IgG1 variants of both Trastuzumab and Pertuzumab. We managed to purify 66 of these to investigate the biophysical characteristics: recombinant protein production, and both Her2 and FcγIIA binding. Our findings revealed combinations that showed improved recombinant antibody production and both antigen and receptor binding kinetics. These findings show the need to rethink antibodies as a whole protein, relooking of the functions of the antibody domains, and the need to include immunoglobulin receptor investigations for effective antibody therapeutics development.
Keywords: FcγIIA; KD; Pertuzumab; Trastuzumab; VH–VL families; complementary determining region grafting; recombinant antibody production; therapeutic antibodies.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

