The Acid-Base Balance and Gender in Inflammation: A Mini-Review
- PMID: 29593728
- PMCID: PMC5854649
- DOI: 10.3389/fimmu.2018.00475
The Acid-Base Balance and Gender in Inflammation: A Mini-Review
Abstract
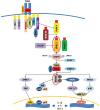
In humans, acid-base balance is crucial to cell homeostasis. Acidosis is observed in numerous inflammatory processes, primarily acute conditions such as sepsis, trauma, or acute respiratory distress where females tend to exhibit better prognosis compared with males. The mechanisms underlying these gender-dependent differences are multiple, probably involving hormonal and genetic factors, particularly the X chromosome. Although pH influences multiple immunological functions, gender differences in acid-base balance have been poorly investigated. In this review, we provide an update on gender differences in human susceptibility to inflammatory diseases. We additionally discuss the potential impact of acid-base balance on the gender bias of the inflammatory response in view of our recent observation that girls present higher neutrophilic inflammation and lower pH with a trend toward better prognosis in severe sepsis. We also highlight the potent role played by endothelial cells in gender differences of inflammation through activation of proton-sensing G protein-coupled receptors.
Keywords: acid–base balance; endothelial cells; gender differences; homeostatic balance; inflammation; mechanisms of inflammatory cascade; monocytes; neutrophils.
Figures
References
-
- Martinez D, Vermeulen M, Trevani A, Ceballos A, Sabatté J, Gamberale R, et al. Extracellular acidosis induces neutrophil activation by a mechanism dependent on activation of phosphatidylinositol 3-kinase/Akt and ERK pathways. J Immunol (2006) 176(2):1163–71.10.4049/jimmunol.176.2.1163 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

