Systemic delivery of selective EP1 and EP3 receptor antagonists attenuates pentylenetetrazole-induced seizures in mice
- PMID: 29593850
- PMCID: PMC5871629
Systemic delivery of selective EP1 and EP3 receptor antagonists attenuates pentylenetetrazole-induced seizures in mice
Abstract
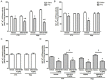
Neuroinflammation plays a major role in brain excitability and may contribute to the development of epilepsy. Prostaglandin E2 (PGE2) is a direct mediator of inflammatory responses and, through EP receptors, plays an important role in neuronal excitability. Pharmacological evidence supports that centrally-administered EP1 and EP3 receptor antagonists reduced acutely evoked seizures in rats. Translation of these findings would benefit from evidence of efficacy with a more clinically relevant route of delivery and validation in another species. In the current study we investigated whether the systemic administration of EP1 and EP3 agonists and antagonists modulate pentylenetetrazole (PTZ)-induced seizures in mice. In addition, it was examined whether these compounds alter Na+, K+-ATPase activity, an enzyme responsible for the homeostatic ionic equilibrium and, consequently, for the resting membrane potential in neurons. While the systemic administration of EP1 and EP3 antagonists (ONO-8713 and ONO-AE3-240, respectively) attenuated, the respective agonists (ONO-DI-004 and ONO-AE-248) potentiated PTZ-induced seizures (all compounds injected at the dose of 10 µg/kg, s.c., 30 min before PTZ challenge). Co-administration of either EP1 or EP3 agonist with the respective antagonists nullified the anticonvulsant effects of EP1/3 receptor blockade. In addition, EP1 and EP3 agonists exacerbated PTZ-induced decrease of Na+, K+-ATPase activity in both cerebral cortex and hippocampus, whereas, EP1 and EP3 antagonists prevented PTZ-induced decrease of Na+, K+-ATPase activity in both structures. Our findings support and extend evidence that EP1 and EP3 receptors may be novel targets for the development of anticonvulsant drugs.
Keywords: EP1 receptor; EP3 receptor; Epilepsy; pentylenetetrazole; prostaglandin E2.
Conflict of interest statement
None.
Figures


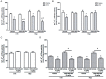
References
-
- Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R, Kroner B, Labiner D, Liow K, Logroscino G, Medina MT, Newton CR, Parko K, Paschal A, Preux PM, Sander JW, Selassie A, Theodore W, Tomson T, Wiebe S ILAE Commission on Epidemiology. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(Suppl 7):2–26. - PubMed
-
- Perucca E, French J, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 2007;6:793–804. - PubMed
-
- Galanopoulou AS, Buckmaster PS, Staley KJ, Moshé SL, Perucca E, Engel J Jr, Löscher W, Noebels JL, Pitkänen A, Stables J, White HS, O’Brien TJ, Simonato M American Epilepsy Society Basic Science Committee and The International League Against Epilepsy Working Group On Recommendations For Preclinical Epilepsy Drug Discovery. Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia. 2012;53:571–82. - PMC - PubMed
-
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–43. - PubMed
LinkOut - more resources
Full Text Sources
