Clinical characteristics and patient-reported outcomes in patients with inadequately controlled rheumatoid arthritis despite ongoing treatment
- PMID: 29593881
- PMCID: PMC5869220
- DOI: 10.1136/rmdopen-2017-000615
Clinical characteristics and patient-reported outcomes in patients with inadequately controlled rheumatoid arthritis despite ongoing treatment
Abstract
Background: Despite the wide array of treatments available for rheumatoid arthritis (RA), some patients continue to report unmet clinical needs. We investigated the extent of inadequate disease control in patients with RA.
Methods: Data were drawn from the Adelphi 2014 RA Disease-Specific Program in France, Germany, Italy, Spain and the UK. Rheumatologists provided patient demographics, comorbidities, satisfaction with RA control and other clinical details. Patients reported their level of satisfaction and completed the EuroQoL 5-Dimensions Health Questionnaire and Work Productivity and Activity Impairment Questionnaire. Patients had been on their current therapy ≥3 months and had 28-joint disease activity scores (DAS28) reported. Adequately controlled (DAS28 ≤3.2) and inadequately controlled (DAS28 >3.2) patient cohorts were compared using univariate tests.
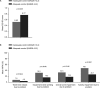
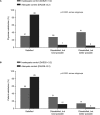
Results: Of 1147 patients, 74% were women, the mean age was 52 years and the mean time since RA diagnosis was 7 years. Twenty-seven percent of patients had inadequately controlled RA, whereas 73% had adequately controlled RA. Inadequately controlled patients were more affected clinically versus adequately controlled patients; 69% vs 13% had moderate/severe RA, the current level of pain was 4.6 vs 2.3, and 67% vs 41% experienced flares, respectively (all p<0.0001). Inadequately controlled patients had higher rates of depression (16% vs 5%; p<0.0001), worse health state, greater work and activity impairment, and lower satisfaction rates among the patients and their physicians than the adequately controlled cohort.
Conclusion: RA was insufficiently controlled in over a quarter of patients despite their current therapy and this had a negative impact on the patients.
Keywords: dmards (biologic); patient perspective.
Conflict of interest statement
Competing interests: PCT has received fees from AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Merck, Pfizer, Sandoz, Biogen and UCB Pharma. RA has received fees from Pfizer. JJGR has received fees from AbbVie, Biogen, Bristol-Myers Squibb, Hospira, Janssen, Merck, Pfizer, Regeneron and UCB Pharma. RC has received fees from AbbVie, MSD, Pfizer, Roche and UCB Pharma. PB has received fees from MSD, Pfizer, Reckitt Benckiser and Roche. ES, RW and JP are employees of Adelphi Real World and were contracted by Pfizer to provide data, input into design of data collection and statistical support for the development of this paper. RV, DS, JA and MT are employees of Pfizer.
Figures
Similar articles
-
Patients with Gout Treated with Conventional Urate-lowering Therapy: Association with Disease Control, Health-related Quality of Life, and Work Productivity.J Rheumatol. 2016 Oct;43(10):1897-1903. doi: 10.3899/jrheum.151199. Epub 2016 Apr 1. J Rheumatol. 2016. PMID: 27036386
-
Outcomes Following Adalimumab Bio-originator to Biosimilar Switch-A Comparison Using Real-world Patient- and Physician-Reported Data in European Countries.Rheumatol Ther. 2023 Apr;10(2):433-445. doi: 10.1007/s40744-022-00526-w. Epub 2023 Jan 12. Rheumatol Ther. 2023. PMID: 36631636 Free PMC article.
-
Tofacitinib for Treating Rheumatoid Arthritis After the Failure of Disease-Modifying Anti-rheumatic Drugs: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Sep;36(9):1063-1072. doi: 10.1007/s40273-018-0639-0. Pharmacoeconomics. 2018. PMID: 29546668 Review.
-
Treatment persistence among patients with rheumatoid disease (RA, AS, PsA) treated with subcutaneous biologics in Germany.Rheumatol Int. 2016 Jan;36(1):143-53. doi: 10.1007/s00296-015-3348-4. Epub 2015 Aug 28. Rheumatol Int. 2016. PMID: 26314368
-
Sarilumab for Previously-Treated Moderate or Severe Rheumatoid Arthritis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Dec;36(12):1427-1437. doi: 10.1007/s40273-018-0677-7. Pharmacoeconomics. 2018. PMID: 29882210 Review.
Cited by
-
Transforming clinical trials in rheumatology: towards patient-centric precision medicine.Nat Rev Rheumatol. 2020 Oct;16(10):590-599. doi: 10.1038/s41584-020-0491-4. Epub 2020 Sep 4. Nat Rev Rheumatol. 2020. PMID: 32887976 Review.
-
Comorbidity burden and clinical characteristics of patients with difficult-to-control rheumatoid arthritis.Clin Rheumatol. 2019 Sep;38(9):2473-2481. doi: 10.1007/s10067-019-04579-1. Epub 2019 May 10. Clin Rheumatol. 2019. PMID: 31076943
-
Levels of satisfaction with rheumatoid arthritis treatment and associated alignment between physicians and patients across Latin America.Clin Rheumatol. 2020 Jun;39(6):1813-1822. doi: 10.1007/s10067-019-04858-x. Epub 2020 Feb 7. Clin Rheumatol. 2020. PMID: 32030635
-
Treatment Satisfaction, Patient Preferences, and the Impact of Suboptimal Disease Control in a Large International Rheumatoid Arthritis Cohort: SENSE Study.Patient Prefer Adherence. 2021 Feb 17;15:359-373. doi: 10.2147/PPA.S289692. eCollection 2021. Patient Prefer Adherence. 2021. PMID: 33633444 Free PMC article.
-
Elevated Serum Levels of YKL-40, YKL-39, and SI-CLP in Patients with Treatment Failure to DMARDs in Patients with Rheumatoid Arthritis.Biomedicines. 2024 Jun 25;12(7):1406. doi: 10.3390/biomedicines12071406. Biomedicines. 2024. PMID: 39061980 Free PMC article.
References
-
- Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care 2012;18(Suppl):S295–302. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
