Reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease
- PMID: 29594027
- PMCID: PMC5861278
- DOI: 10.21037/tau.2017.12.29
Reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease
Abstract
Background: The fear of undergrading prostate cancer (PCa) in men on active surveillance (AS) have led to strict criteria for monitoring, which have resulted in good long-term cancer-specific survival, proving the safety of this approach. Reducing undergrading, MRI-targeted biopsies are increasingly used in men with low-risk disease despite their undefined role yet. The objective of this study is to investigate the rate of upgrading using MRI-targeted biopsies in men with low-risk disease on AS, stratified on the basis of PI-RADS and PSA-density, with the aim to reduce potential unnecessary repeat biopsy procedures.
Methods: A total of 331 men were prospectively enrolled following the MRI-PRIAS protocol. MR imaging was according to Prostate Imaging Reporting and Data System (PI-RADSv2) guidelines. Suspicious MRI lesions (PI-RADS 3-5) were additionally targeted by MRI-TRUS fusion biopsies. Outcome measure was upgrading to Gleason score (GS) ≥3+4 with MRI-targeted biopsies, stratified for PI-RADS and PSA-density.
Results: In total, 25% (82/331) of men on AS showed upgrading from GS 3+3. Only 3% (11/331) was upgraded to GS ≥8. In 60% (198/331) a suspicious MRI lesion was identified, but in only 41% (82/198) of men upgrading was confirmed. PI-RADS 3, 4 and 5 categorized index lesions, showed upgrading in 30%, 34% and 66% of men, respectively. Stratification to PI-RADS 4-5, instead of PI-RADS 3-5, would have missed a small number of high volume Gleason 4 PCa in PI-RADS 3 category. However, further stratification into PI-RADS 3 lesions and PSA-density <0.15 ng/mL2 could result in a safe targeted biopsy reduction of 36% in this category, without missing any upgrades.
Conclusions: Stratification with the combination of PI-RADS and PSA-density may reduce unnecessary additional MRI biopsy testing. Overall, the high rate of detected upgrading in men on AS may result in an unintended tightening of continuing in AS. Since patients, included under current AS criteria showed extremely favorable outcome, there might be no need to further restrict continuing on AS with MRI and targeted biopsies.
Keywords: MRI-guided targeted biopsy; PI-RADS; PSA density; Prostate cancer (PCa); biopsy; magnetic resonance imaging (MRI); risk stratification.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

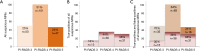





Similar articles
-
Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer.BJU Int. 2017 Oct;120(4):511-519. doi: 10.1111/bju.13836. Epub 2017 Apr 4. BJU Int. 2017. PMID: 28267899
-
Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: reasons for targeted biopsy failure.BJU Int. 2016 Jul;118(1):35-43. doi: 10.1111/bju.13327. Epub 2015 Oct 20. BJU Int. 2016. PMID: 26384851
-
Evaluating the performance of existing tools to predict clinically significant prostate cancer in men with indeterminate lesions on biparametric MRI and development of a novel multiplex model: a prospective cohort study.EClinicalMedicine. 2025 Apr 3;82:103191. doi: 10.1016/j.eclinm.2025.103191. eCollection 2025 Apr. EClinicalMedicine. 2025. PMID: 40242565 Free PMC article.
-
MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions?Transl Androl Urol. 2018 Feb;7(1):70-82. doi: 10.21037/tau.2017.12.31. Transl Androl Urol. 2018. PMID: 29594022 Free PMC article. Review.
-
Multiparametric MRI in detection and staging of prostate cancer.Dan Med J. 2017 Feb;64(2):B5327. Dan Med J. 2017. PMID: 28157066 Review.
Cited by
-
Personalized risk-adapted models in prostate cancer during active surveillance using MRI-a narrative review.Eur Radiol. 2025 Apr 4. doi: 10.1007/s00330-025-11518-z. Online ahead of print. Eur Radiol. 2025. PMID: 40185922 Review.
-
Optimal PSA density threshold for prostate biopsy in benign prostatic obstruction patients with elevated PSA levels but negative MRI findings.BMC Urol. 2025 Mar 3;25(1):42. doi: 10.1186/s12894-025-01719-5. BMC Urol. 2025. PMID: 40033313 Free PMC article.
-
Evaluation of blood and urine based biomarkers for detection of clinically-significant prostate cancer.Prostate Cancer Prostatic Dis. 2025 Mar;28(1):45-55. doi: 10.1038/s41391-024-00840-0. Epub 2024 Jun 10. Prostate Cancer Prostatic Dis. 2025. PMID: 38858447 Free PMC article. Review.
-
Computer-Aided Diagnosis in Multiparametric MRI of the Prostate: An Open-Access Online Tool for Lesion Classification with High Accuracy.Cancers (Basel). 2020 Aug 21;12(9):2366. doi: 10.3390/cancers12092366. Cancers (Basel). 2020. PMID: 32825612 Free PMC article.
-
Evaluating the Utility of Prostate-Specific Antigen Density in Risk Stratification of PI-RADS 3 Peripheral Zone Lesions on Non-Contrast-Enhanced Prostate MRI: An Exploratory Single-Institution Study.Cureus. 2023 Jul 4;15(7):e41369. doi: 10.7759/cureus.41369. eCollection 2023 Jul. Cureus. 2023. PMID: 37546087 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
