Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis
- PMID: 29594037
- PMCID: PMC5857934
- DOI: 10.3389/fonc.2018.00055
Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis
Abstract
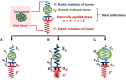
Solid tumors are characterized by an abnormal stroma that contributes to the development of biomechanical abnormalities in the tumor microenvironment. In particular, these abnormalities include an increase in matrix stiffness and an accumulation of solid stress in the tumor interior. So far, it is not clearly defined whether matrix stiffness and solid stress are strongly related to each other or they have distinct roles in tumor progression. Moreover, while the effects of stiffness on tumor progression are extensively studied compared to the contribution of solid stress, it is important to ascertain the biological outcomes of both abnormalities in tumorigenesis and metastasis. In this review, we discuss how each of these parameters is evolved during tumor growth and how these parameters are influenced by each other. We further review the effects of matrix stiffness and solid stress on the proliferative and metastatic potential of cancer and stromal cells and summarize the in vitro experimental setups that have been designed to study the individual contribution of these parameters.
Keywords: externally applied stress; extracellular matrix; fibroblasts; growth-induced stress; in vitro models.
Figures

Similar articles
-
The emergence of solid stress as a potent biomechanical marker of tumour progression.Emerg Top Life Sci. 2018 Dec 21;2(5):739-749. doi: 10.1042/ETLS20180049. Emerg Top Life Sci. 2018. PMID: 33530664
-
Cancer-associated fibroblasts promote oral squamous cell carcinoma progression through LOX-mediated matrix stiffness.J Transl Med. 2021 Dec 20;19(1):513. doi: 10.1186/s12967-021-03181-x. J Transl Med. 2021. PMID: 34930321 Free PMC article.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
-
The role of matrix stiffness in cancer stromal cell fate and targeting therapeutic strategies.Acta Biomater. 2022 Sep 15;150:34-47. doi: 10.1016/j.actbio.2022.08.005. Epub 2022 Aug 7. Acta Biomater. 2022. PMID: 35948177 Review.
-
Molecular insights into prostate cancer progression: the missing link of tumor microenvironment.J Urol. 2005 Jan;173(1):10-20. doi: 10.1097/01.ju.0000141582.15218.10. J Urol. 2005. PMID: 15592017 Review.
Cited by
-
Pancreatic Cancer Presents Distinct Nanomechanical Properties During Progression.Ann Biomed Eng. 2023 Jul;51(7):1602-1615. doi: 10.1007/s10439-023-03168-3. Epub 2023 Feb 22. Ann Biomed Eng. 2023. PMID: 36813931
-
Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling.Sci Rep. 2020 Jan 9;10(1):50. doi: 10.1038/s41598-019-55687-6. Sci Rep. 2020. PMID: 31919369 Free PMC article.
-
Effect of Compressive Stress in Tumor Microenvironment on Malignant Tumor Spheroid Invasion Process.Int J Mol Sci. 2022 Jun 25;23(13):7091. doi: 10.3390/ijms23137091. Int J Mol Sci. 2022. PMID: 35806095 Free PMC article.
-
The impact of tumor microenvironment: unraveling the role of physical cues in breast cancer progression.Cancer Metastasis Rev. 2024 Jun;43(2):823-844. doi: 10.1007/s10555-024-10166-x. Epub 2024 Jan 19. Cancer Metastasis Rev. 2024. PMID: 38238542 Free PMC article. Review.
-
A Simple Method to Test Mechanical Strain on Epithelial Cell Monolayers Using a 3D-Printed Stretcher.Methods Mol Biol. 2021;2367:235-247. doi: 10.1007/7651_2020_314. Methods Mol Biol. 2021. PMID: 32789778 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

