In vivo Effects of Repeated Thyronamine Administration in Male C57BL/6J Mice
- PMID: 29594048
- PMCID: PMC5836237
- DOI: 10.1159/000481856
In vivo Effects of Repeated Thyronamine Administration in Male C57BL/6J Mice
Abstract
Objectives: Thyronamines are decarboxylated and deiodinated metabolites of thyroid hormones (THs). Of all possible thyronamine variants, only 3-iodothyronamine (3-T1AM) and iodine-free thyronamine (T0AM) have been detected in vivo. While intensive research has been done on the (patho-)physiological action of 3-T1AM, the role of T0AM has been studied less intensively.
Study design: We determined whether a single pharmacological dose (50 mg/kg, i.p.) or repeated administration (5 mg/kg/day, i.p., for 7 days) of T0AM affects metabolism, cardiovascular function, or thermoregulation in male C57BL/6J mice. Since selenium (Se) is important for proper TH function and Se metabolism is affected by TH, we additionally analyzed Se concentrations in liver, serum, and kidney using total reflection X-ray analysis.
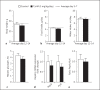
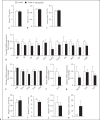
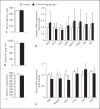
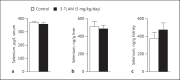
Results: A single injection of T0AM had no effect on heart rate, temperature, or activity as assessed by radio telemetry. Likewise, daily administration of T0AM did not alter body weight, food or water intake, heart rate, blood pressure, brown adipose tissue thermogenesis, or body temperature, and no significant differences in hepatic glycogen content or mRNA expression of genes involved in cardiovascular function or metabolic control were determined. Also, the X-ray analysis of Se concentrations revealed no significant changes. However, hepatic T0AM was significantly increased in the treated animals.
Conclusions: In summary, our data demonstrate that T0AM elicits no obvious metabolic, cardiovascular, or thermoregulatory activities in mice. As T0AM does also not interfere with TH or Se metabolism, we conclude that the deiodination of 3-T1AM to T0AM constitutes an efficient inactivation mechanism, terminating the actions of the more powerful precursor.
Keywords: 3-Iodothyronamine; Brown adipose tissue; Heart rate; Metabolism; Thermoregulation; Thyroid hormone; Trace elements.
Figures


References
-
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nature medicine. 2004;10:638–642. - PubMed
-
- Hoefig CS, Köhrle J, Brabant G, Dixit K, Yap B, Strasburger CJ, Wu Z. Evidence for extrathyroidal formation of 3-iodothyronamine in humans as provided by a novel monoclonal antibody-based chemiluminescent serum immunoassay. J Clin Endocrinol Metab. 2011;96:1864–1872. - PubMed
-
- Liggett SB. The two-timing thyroid. Nat Med. 2004;10:582–583. - PubMed
-
- Schanze N, Jacobi SF, Rijntjes E, Mergler S, Del Olmo M, Hoefig CS, Khajavi N, Lehmphul I, Biebermann H, Mittag J, Kohrle J. 3-Iodothyronamine decreases expression of genes involved in iodide metabolism in mouse thyroids and inhibits iodide uptake in PCCL3 thyrocytes. Thyroid. 2017;27:11–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

