Metazoan Parasite Vaccines: Present Status and Future Prospects
- PMID: 29594064
- PMCID: PMC5859119
- DOI: 10.3389/fcimb.2018.00067
Metazoan Parasite Vaccines: Present Status and Future Prospects
Abstract
Eukaryotic parasites and pathogens continue to cause some of the most detrimental and difficult to treat diseases (or disease states) in both humans and animals, while also continuously expanding into non-endemic countries. Combined with the ever growing number of reports on drug-resistance and the lack of effective treatment programs for many metazoan diseases, the impact that these organisms will have on quality of life remain a global challenge. Vaccination as an effective prophylactic treatment has been demonstrated for well over 200 years for bacterial and viral diseases. From the earliest variolation procedures to the cutting edge technologies employed today, many protective preparations have been successfully developed for use in both medical and veterinary applications. In spite of the successes of these applications in the discovery of subunit vaccines against prokaryotic pathogens, not many targets have been successfully developed into vaccines directed against metazoan parasites. With the current increase in -omics technologies and metadata for eukaryotic parasites, target discovery for vaccine development can be expedited. However, a good understanding of the host/vector/pathogen interface is needed to understand the underlying biological, biochemical and immunological components that will confer a protective response in the host animal. Therefore, systems biology is rapidly coming of age in the pursuit of effective parasite vaccines. Despite the difficulties, a number of approaches have been developed and applied to parasitic helminths and arthropods. This review will focus on key aspects of vaccine development that require attention in the battle against these metazoan parasites, as well as successes in the field of vaccine development for helminthiases and ectoparasites. Lastly, we propose future direction of applying successes in pursuit of next generation vaccines.
Keywords: OMICS techniques; antigen identification; parasite control; parasites; systems biology; vaccine development; vaccines.
Figures
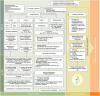
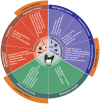
References
-
- Abel I., Da Cunha N. C., Rangel C. P., Do Nascimento Corrêa F., Da Fonseca A. H. (2016). Artificial feeding of partially engorged Amblyomma sculptum females through capillaries. Braz. J. Veterin. Med. 38, 211–217. Available online at: http://rbmv.org/index.php/BJVM/article/view/300
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

