Detergent Desorption of Membrane Proteins Exhibits Two Kinetic Phases
- PMID: 29595981
- PMCID: PMC5908730
- DOI: 10.1021/acs.jpclett.8b00549
Detergent Desorption of Membrane Proteins Exhibits Two Kinetic Phases
Abstract
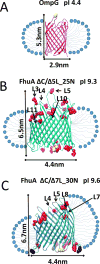
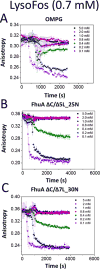
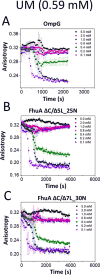
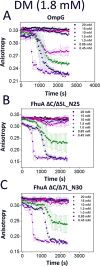
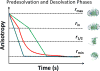
Gradual dissociation of detergent molecules from water-insoluble membrane proteins culminates in protein aggregation. However, the time-dependent trajectory of this process remains ambiguous because the signal-to-noise ratio of most spectroscopic and calorimetric techniques is drastically declined by the presence of protein aggregates in solution. We show that by using steady-state fluorescence polarization (FP) spectroscopy the dissociation of the protein-detergent complex (PDC) can be inspected in real time at detergent concentrations below the critical micelle concentration. This article provides experimental evidence of the coexistence of two distinct phases of the dissociations of detergent monomers from membrane proteins. We first noted a slow detergent predesolvation process, which was accompanied by a relatively modest change in the FP anisotropy, suggesting a small number of dissociated detergent monomers from the proteomicelles. This predesolvation phase was followed by a fast detergent desolvation process, which was highlighted by a major alteration in the FP anisotropy. The durations and rates of these phases were dependent on both the detergent concentration and the interfacial PDC interactions. Further development of this approach might lead to the creation of a new semiquantitative method for the assessment of the kinetics of association and dissociation of proteomicelles.
Figures

Similar articles
-
High-Throughput Screening of Protein-Detergent Complexes Using Fluorescence Polarization Spectroscopy.Curr Protoc Protein Sci. 2019 Sep;97(1):e96. doi: 10.1002/cpps.96. Curr Protoc Protein Sci. 2019. PMID: 31517448 Free PMC article.
-
Kinetics of Membrane Protein-Detergent Interactions Depend on Protein Electrostatics.J Phys Chem B. 2018 Oct 18;122(41):9471-9481. doi: 10.1021/acs.jpcb.8b07889. Epub 2018 Oct 5. J Phys Chem B. 2018. PMID: 30251852 Free PMC article.
-
Interrogating Detergent Desolvation of Nanopore-Forming Proteins by Fluorescence Polarization Spectroscopy.Anal Chem. 2017 Aug 1;89(15):8013-8020. doi: 10.1021/acs.analchem.7b01339. Epub 2017 Jul 10. Anal Chem. 2017. PMID: 28650154 Free PMC article.
-
The mechanism of detergent solubilization of lipid bilayers.Biophys J. 2013 Jul 16;105(2):289-99. doi: 10.1016/j.bpj.2013.06.007. Biophys J. 2013. PMID: 23870250 Free PMC article. Review.
-
Interaction of membrane proteins and lipids with solubilizing detergents.Biochim Biophys Acta. 2000 Nov 23;1508(1-2):86-111. doi: 10.1016/s0304-4157(00)00010-1. Biochim Biophys Acta. 2000. PMID: 11090820 Review.
Cited by
-
High-Throughput Screening of Protein-Detergent Complexes Using Fluorescence Polarization Spectroscopy.Curr Protoc Protein Sci. 2019 Sep;97(1):e96. doi: 10.1002/cpps.96. Curr Protoc Protein Sci. 2019. PMID: 31517448 Free PMC article.
-
Kinetics of the multitasking high-affinity Win binding site of WDR5 in restricted and unrestricted conditions.Biochem J. 2021 Jun 11;478(11):2145-2161. doi: 10.1042/BCJ20210253. Biochem J. 2021. PMID: 34032265 Free PMC article.
-
Kinetics of Membrane Protein-Detergent Interactions Depend on Protein Electrostatics.J Phys Chem B. 2018 Oct 18;122(41):9471-9481. doi: 10.1021/acs.jpcb.8b07889. Epub 2018 Oct 5. J Phys Chem B. 2018. PMID: 30251852 Free PMC article.
-
Protein Ligand-Induced Amplification in the 1/f Noise of a Protein-Selective Nanopore.Langmuir. 2020 Dec 22;36(50):15247-15257. doi: 10.1021/acs.langmuir.0c02498. Epub 2020 Dec 13. Langmuir. 2020. PMID: 33307706 Free PMC article.
References
-
- Pocanschi CL, Popot JL, Kleinschmidt JH. Folding and stability of outer membrane protein A (OmpA) from Escherichia coli in an amphipathic polymer, amphipol A8-35. Eur Biophys J. 2013;42(2-3):103–118. - PubMed
-
- Sadaf A, Cho KH, Byrne B, Chae PS. Amphipathic agents for membrane protein study. Methods Enzymol. 2015;557:57–94. - PubMed
-
- Pollock NL, Satriano L, Zegarra-Moran O, Ford RC, Moran O. Structure of wild type and mutant F508del CFTR: A small-angle X-ray scattering study of the protein-detergent complexes. J Struct Biol. 2016;194(1):102–11. - PubMed
-
- Li J, Qiu XJ. Quantification of membrane protein self-association with a high-throughput compatible fluorescence assay. Biochemistry. 2017;56(14):1951–1954. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

